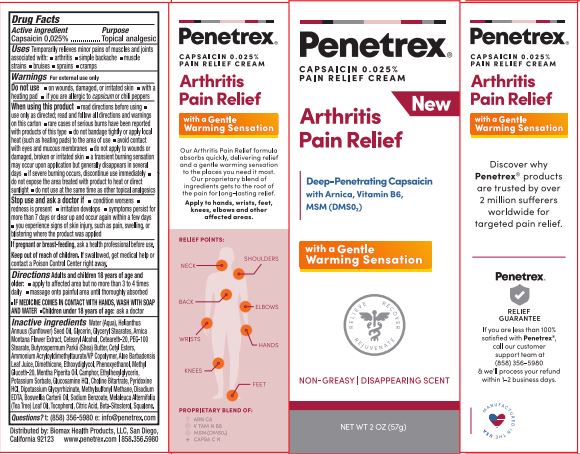
Uses
Temporarily relieves minor pains of muscles and joints associated with arthritis,simple backache,muscle strains,bruises,sprains,cramps
Do not use
on wounds, damaged, or irritated skin
with a heating pad
if you are alergic to capsicum or chili peppers
When using this product
Read directions befbre using
use only as directed; read and follow all directions and warnings on this carton rare cases of serious bums have been reported with products of this type
do not bandage tightly or apply local heat (such as heating pads) to the area of use
avoid contact with eyes and mucous membranes
do not apply to wounds or damaged, broken or irritated skin
a transient burning sensation may occur upon application but generally disappears in several days
if severe burning occurs, discontinue use immediately
do not expose the area treated With product to heat or direct sunlight
do not use at the same time as other topical analgesics,
Stop use and ask a doctor if
condition worsens
redness is present
irritation develops
symptoms persist for more than 7 days or clear up and occur again within a few days
you experience signs of skin injury, such as pain, swelling, or blistering where the product was applied
Keep out of reach of children.
If swalowed, get medical help or contact a Poison Control Center right away.
Directions:
Adults and children 18 years of age and older:
apply to affected area but no more than 3 to 4 times daily
massage onto painful area until thoroughly absorbed
IF MEDICINE COMES IN CONTACT WITH HANDS, WASH WITH SOAP AND WATER
Children under 18 years of age: ask a doctor
Inactive ingredients:
Water (Aqua),Helanthus Annuus (Sunflower) Seed Oil, Glycerin,GlycerylStearates, Amica Montana Flower Extract,
Cetearyl Alcohol,Ceteareth-20,PEG-100 Stearate, Butyrospermum Parkii (Shea) Buffer,Cetyl Esters,Ammonium Acryloyldimethyltaurate/VP Copolymer, Aloe Barbadensis Leaf Juice,Dimethicone, Ethoxydiglycol, Phenoxyethanol,
Methyl Gluceth-20,Menthe Piperita Oil,Camphor,Ethylhexylglycerin,Potassium Sorbate, Glucosamine HCI,Choline Bitartrate,
Pyridoxine HCL, Glycyrrhizinate Dipotassium, Methyl sulfonyl Methane,Disodium EDTA, Boswellia carterii oil,
Sodium Benzoate, Melaleuca Alternifolia (Tea Tree) Leaf Oil, Tocopherol, Citric Acid,Beta-Sitosterol, Squalene.