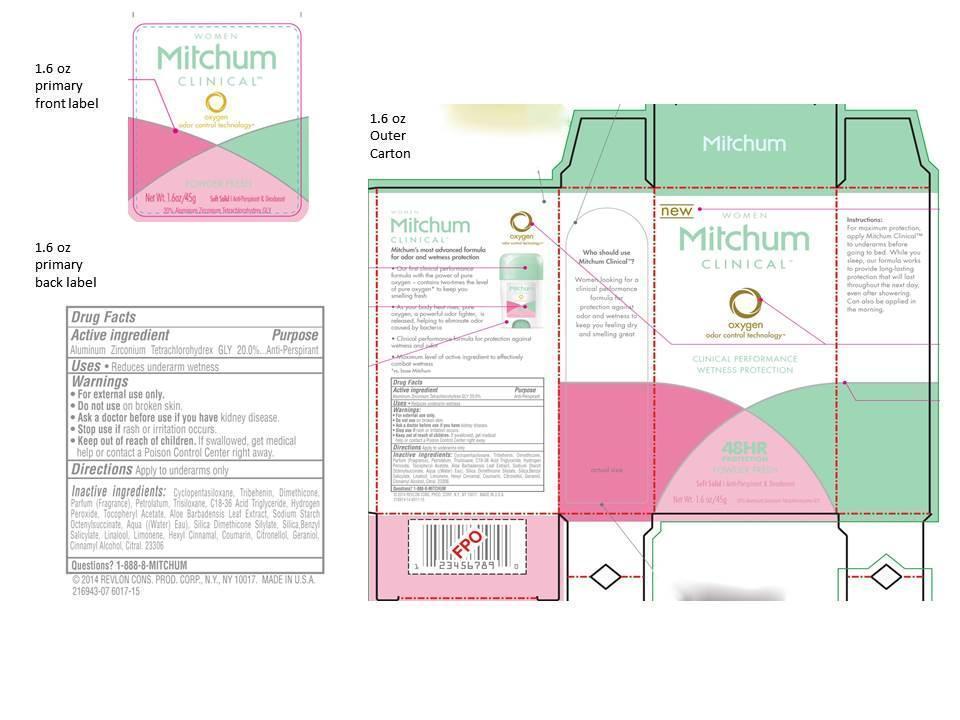
Warnings:
For external use only.
Do not use on broken skin
Ask a doctor before use if you have kidney disease
Stop use if rash or irriation occurs
Keep out of reach of children.
If swallowed, get medical help or contact a Poison Control Center right away.
Inactive Ingredients
cyclopentasiloxane, tribehenin, dimethicone, parfum (fragrance), petrolatum, trisiloxane, c18-36 acid triglyceride, hydrogen peroxide, tocopheryl acetate, aloe barbadensis leaf extract, sodium starch octenylsuccinate, aqua((water) eau), silica dimethicone silylate, silica, benzyl salicylate, linalool, limonene, hexyl cinnamal, coumarin, citronellol, geraniol, cinnamyl alcohol, citral