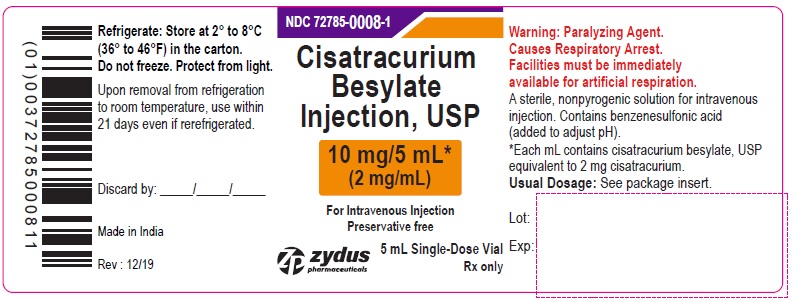
Principal Display Panel – Container Label (10 mg/5 mL)
NDC 72785-0008-1
Cisatracurium Besylate Injection, USP
10 mg/5 mL*
(2 mg/mL)
WARNING: Paralyzing Agent
For Intravenous Injection
Preservative free
5 mL Single-Dose Vial
Rx only
Zydus Pharmaceuticals
Principal Display Panel – Container Label (200 mg/20 mL)
NDC 72785-0009-1
Cisatracurium Besylate Injection, USP
200 mg/20 mL*
(10 mg/mL)
WARNING: Paralyzing Agent
For ICU use only.
For Intravenous Injection
Preservative free
20 mL Single-Dose Vial
Rx only
Zydus Pharmaceuticals

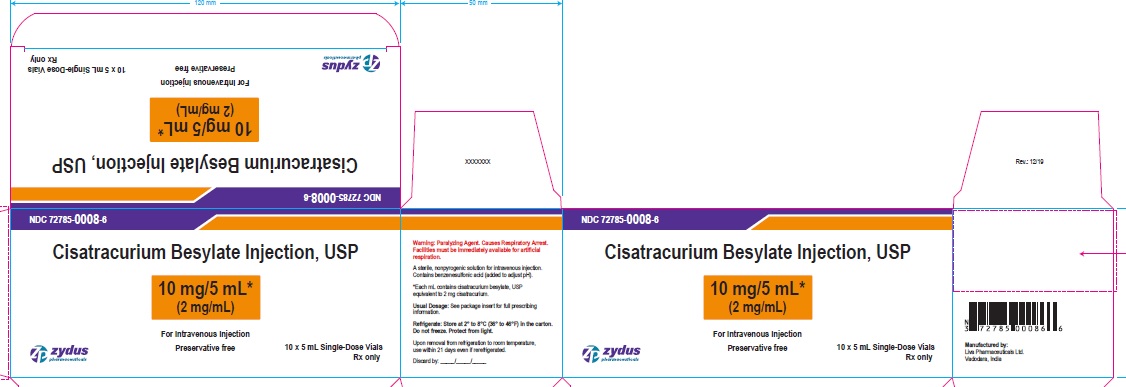
Principal Display Panel – Carton Label (10 mg/5 mL)
NDC 72785-0008-6
Cisatracurium Besylate Injection, USP
10 mg/5 mL*
(2 mg/mL)
WARNING: Paralyzing Agent
For Intravenous Injection
Preservative free
10 x 5 mL Single-Dose Vials
Rx only
Zydus Pharmaceuticals
Principal Display Panel – Carton Label (200 mg/20 mL)
NDC 72785-0009-6
Cisatracurium Besylate Injection, USP
200 mg/20 mL*
(10 mg/mL)
WARNING: Paralyzing Agent
For ICU use only.
For Intravenous Injection
Preservative free
10 x 20 mL Single-Dose Vials
Rx only
Zydus Pharmaceuticals
