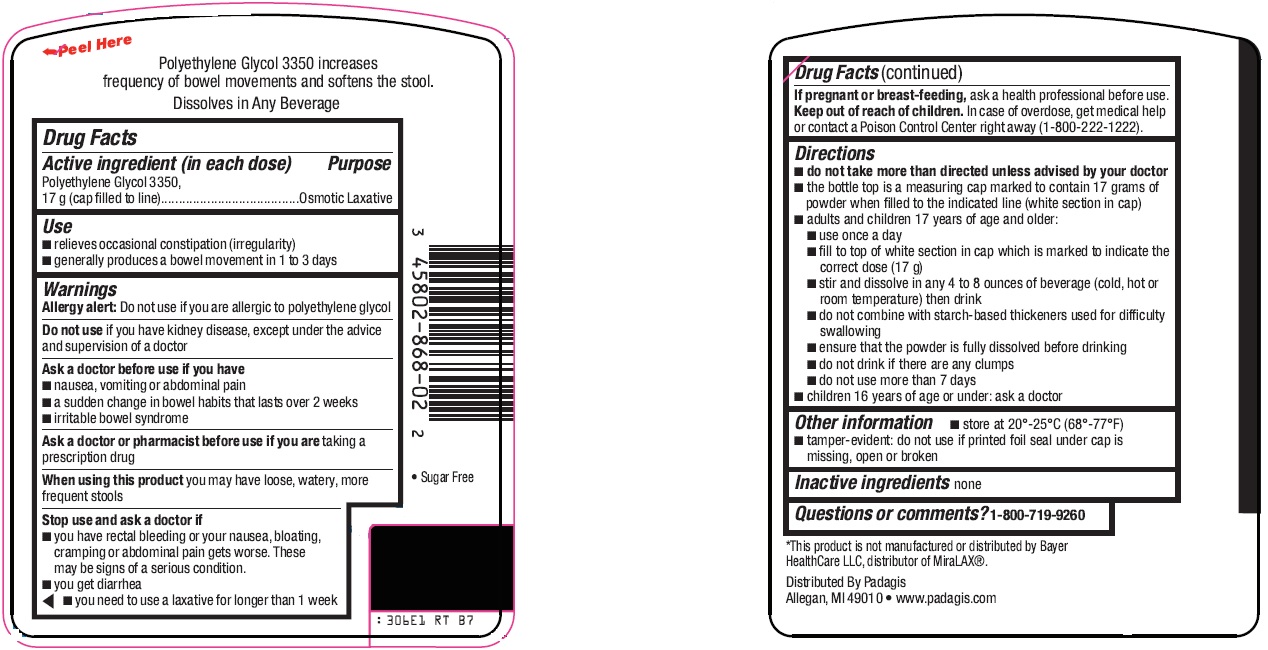
Active ingredient (in each dose)
Polyethylene Glycol 3350, 17 g (cap filled to line)
Use
- •
- relieves occasional constipation (irregularity)
- •
- generally produces a bowel movement in 1 to 3 days
Warnings
Allergy alert: Do not use if you are allergic to polyethylene glycol
Do not use
if you have kidney disease, except under the advice and supervision of a doctor
Ask a doctor before use if you have
- •
- nausea, vomiting or abdominal pain
- •
- a sudden change in bowel habits that lasts over 2 weeks
- •
- irritable bowel syndrome
Ask a doctor or pharmacist before use if you are
taking a prescription drug
When using this product
you may have loose, watery, more frequent stools
Stop use and ask a doctor if
- •
- you have rectal bleeding or your nausea, bloating, cramping or abdominal pain gets worse. These may be signs of a serious condition.
- •
- you get diarrhea
- •
- you need to use a laxative for longer than 1 week
If pregnant or breast-feeding,
ask a health professional before use.
Keep out of reach of children.
In case of overdose, get medical help or contact a Poison Control Center right away (1-800-222-1222).
Directions
- •
-
do not take more than directed unless advised by your doctor
- •
- the bottle top is a measuring cap marked to contain 17 grams of powder when filled to the indicated line (white section in cap)
- •
- adults and children 17 years of age and older:
- •
- use once a day
- •
- fill to top of white section in cap which is marked to indicate the correct dose (17 g)
- •
- stir and dissolve in any 4 to 8 ounces of beverage (cold, hot or room temperature) then drink
- •
- do not combine with starch-based thickeners used for difficulty swallowing
- •
- ensure that the powder is fully dissolved before drinking
- •
- do not drink if there are any clumps
- •
- do not use more than 7 days
- •
- children 16 years of age or under: ask a doctor
Other information
- •
- store at 20°-25°C (68°-77°F)
- •
- tamper-evident: do not use if printed foil seal under cap is missing, open or broken
Inactive ingredients
none
Questions or comments?
1-800-719-9260
Principal Display Panel
Compare to MiraLAX® active ingredient
Polyethylene Glycol 3350
Powder for Solution
Osmotic Laxative
- Relieves Occasional Constipation/Irregularity
- Softens Stool
Unflavored Powder
Grit Free
NET WT 8.3 OZ (238 g)
14 Once-Daily Doses