ARNICA- cvs arnica montana gel
CVS Health
Disclaimer: This homeopathic product has not been evaluated by the Food and Drug Administration for safety or efficacy. FDA is not aware of scientific evidence to support homeopathy as effective.
----------
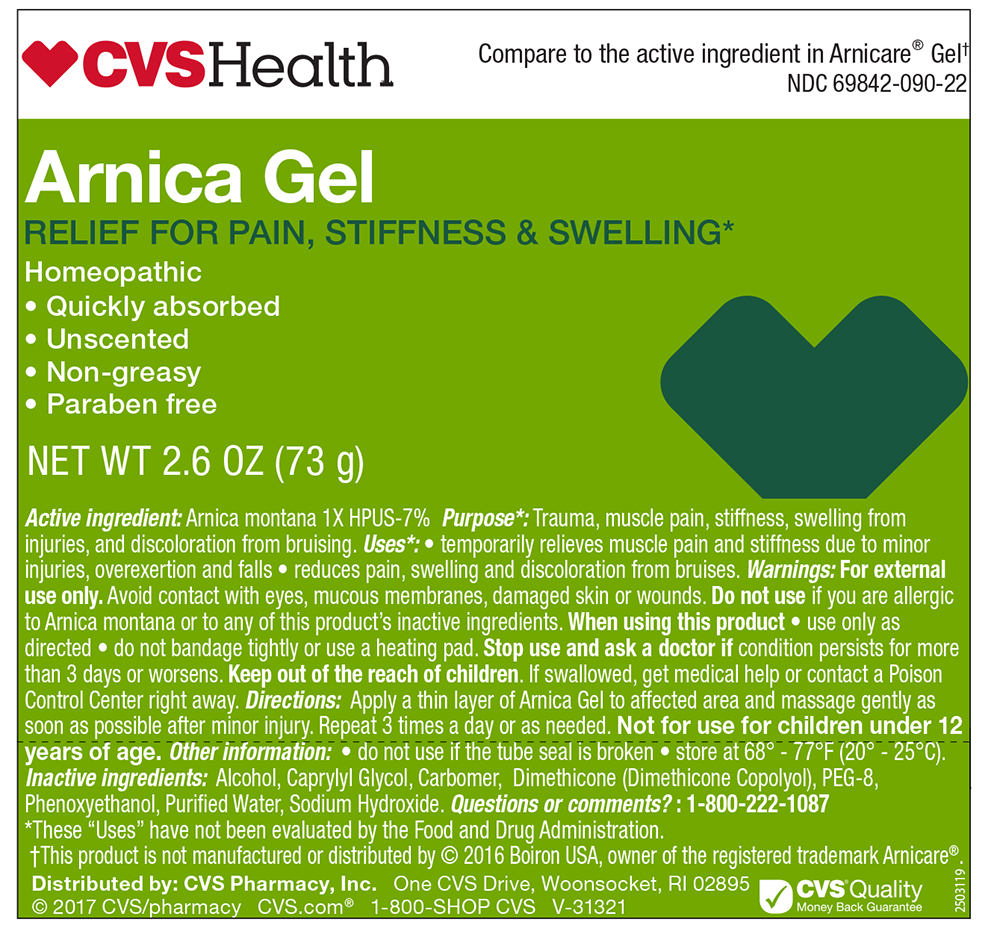
CVS Health Arnica Gel
The letters HPUS indicate that this ingredient is offically included in the Homoepathic Pharmacopoeia of the United States.
Uses
- temporarily relieves muscle pain and stiffness due to minor injuries
- overexcertion and falls
- reduced pain, swelling and discoloration from bruises
Warnings
For external use only.Avoid contact with eyes, mucous membranes, damaged skin or wounds. Do not use if you are allergic to Arnica montana or to any of this product's inactive ingredients.
Keep out of the reach of children. If swallowed, get medical help or contact Poison Control Center right away.Not for use of children 12 years of age
Directions
Apply a thin layer of Arnica Gel to the affected area and massage gently as soon as possible after the minor injury. Repeat 3 times a day or as needed.
Inactive ingredients
Alcohol, Caprylyl Glycol, Carbomer, Dimethicone ( Dimethicone Copolyol), PEG-8, Phenoxyethanol, Purified Water, Sodium Hydroxide
| ARNICA
cvs arnica montana gel |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - CVS Health (062312574) |
| Registrant - Sheffield Pharmaceuticals LLC (151177797) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Sheffield Pharmaceuticals LLC | 151177797 | manufacture(69842-090) | |