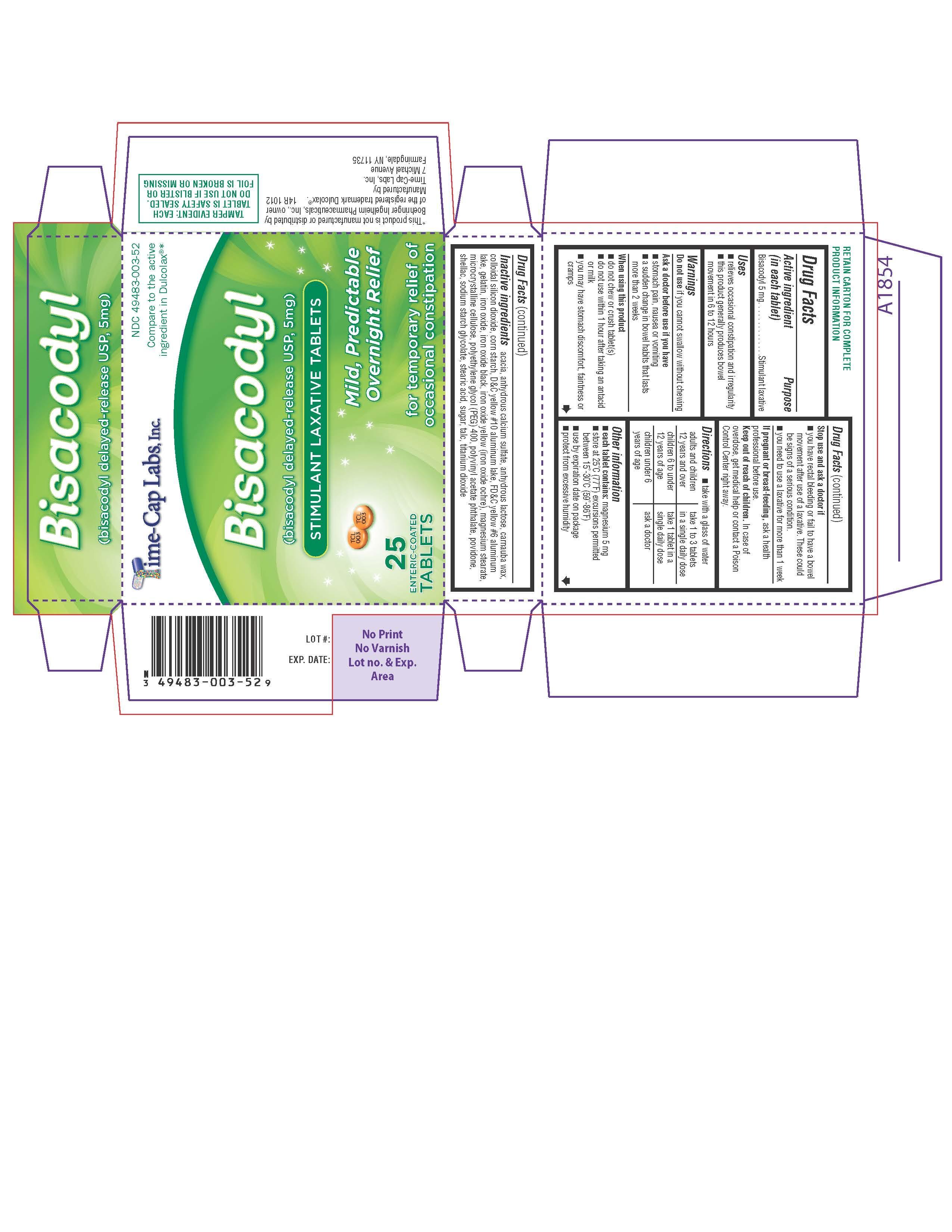
Acadia, Anhydrous Calcium Sulfate, Anhydrous Lactose, Carnauba Wax, Colloidal Silicon Dioxide, Corn Starch, D&C Yellow #10 Aluminum Lake, FD&C yellow #6 aluminum lake, Gelatin, Iron Oxide, Iron Oxide Black, Iron Oxide Yellow(Iron Oxide Ochre), Magnesium Stearate, Microcrystalline Cellulose, Polyethylene Glycol (PEG) 400, Polyvinyl Acetate Phthalates, Povidone, Shellac, Sodium Starch Glycolate, Stearic Acid, Sugar, Talc, Titanium Dioxide
Keep out of Reach of Children: In case of overdose, get medical help or contact a Poison Control Center right away.
Uses:
- relieves occasional constipation and irregularity
- this product generally produces a bowel movement in 6 to 12 hours
Warning: Do not use if you cannot swallow without chewing.
Ask a doctor before use if you have: stomach pain, nausea or vomiting; A sudden change in bowel habits that lasts more than two weeks.
When using this product: Do not chew or crush tablet(s); Do not use within 1 hour after taking an antacid or milk; Do not use this product if you have stomach discomfort, faintness or cramps.