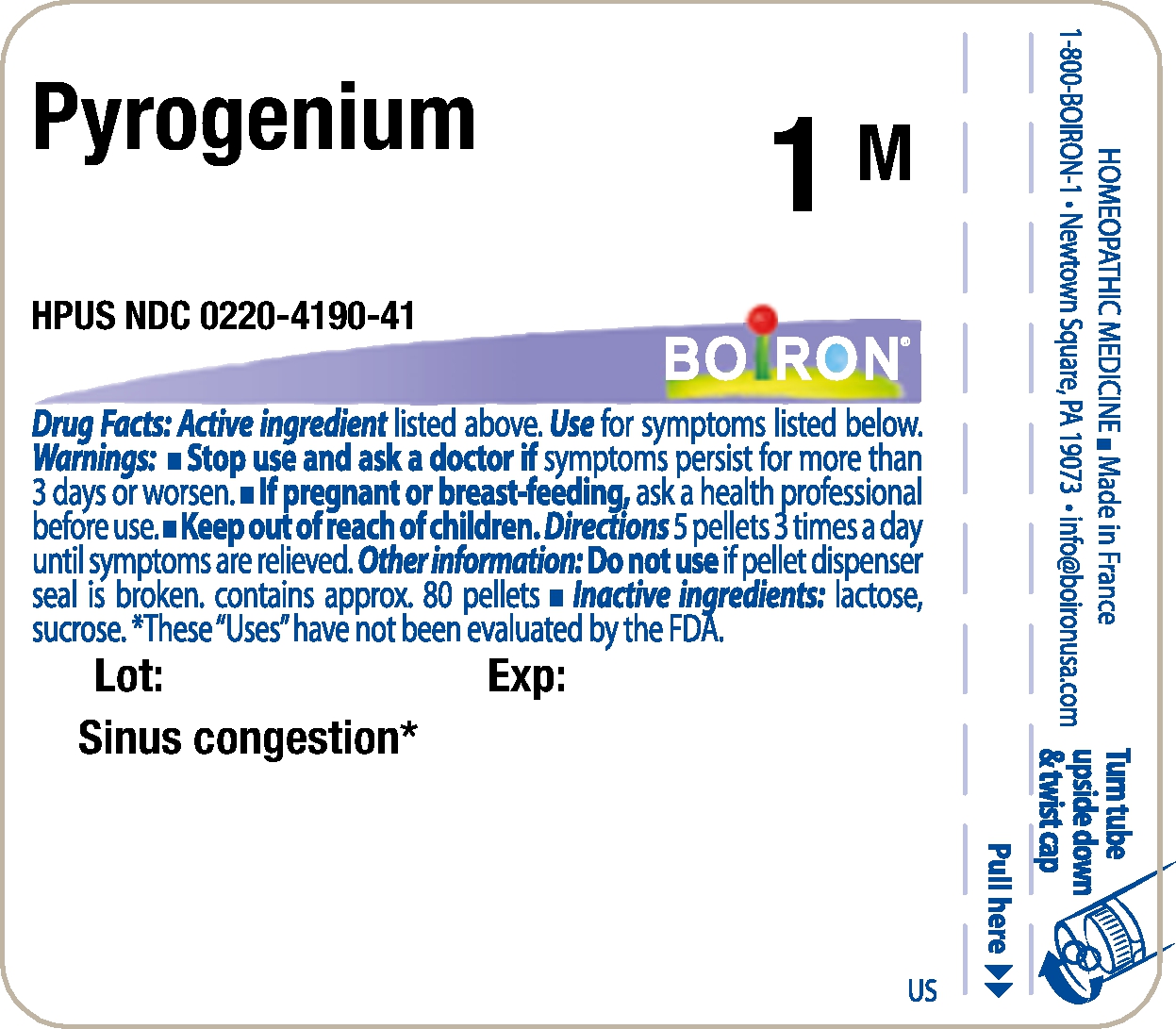
PYROGENIUM- rancid beef pellet
Laboratoires Boiron
Disclaimer: This homeopathic product has not been evaluated by the Food and Drug Administration for safety or efficacy. FDA is not aware of scientific evidence to support homeopathy as effective.
----------
Active Ingredients
Pyrogenium HPUS
6C, 9C, 12C, 15C, 30C, 200CK, 1M
Warnings
Stop use and ask a doctor if
symptoms persist for more than 3 days or worsen.
If pregnant or breast-feeding,
ask a health professional before use.
Keep out of reach of children.
Directions
5 pellets 3 times a day until symptoms are relieved.
Other information
Do not use if pellet dispenser seal is broken.
contains approx. 80 pellets
Inactive ingredients
lactose, sucrose
Questions, Comments?
1-800-BOIRON-1
Newtown Square, PA 19073-3267
info@boironusa.com
*
These "Uses" have not been evaluated by the FDA.
Principle Display Panel - Pyrogenium HPUS 6C

Principle Display Panel - Pyrogenium HPUS 9C

Principle Display Panel - Pyrogenium HPUS 12C

Principle Display Panel - Pyrogenium HPUS 15C

Principle Display Panel - Pyrogenium HPUS 30C

Principle Display Panel - Pyrogenium HPUS 200CK

Principle Display Panel - Pyrogenium HPUS 1M