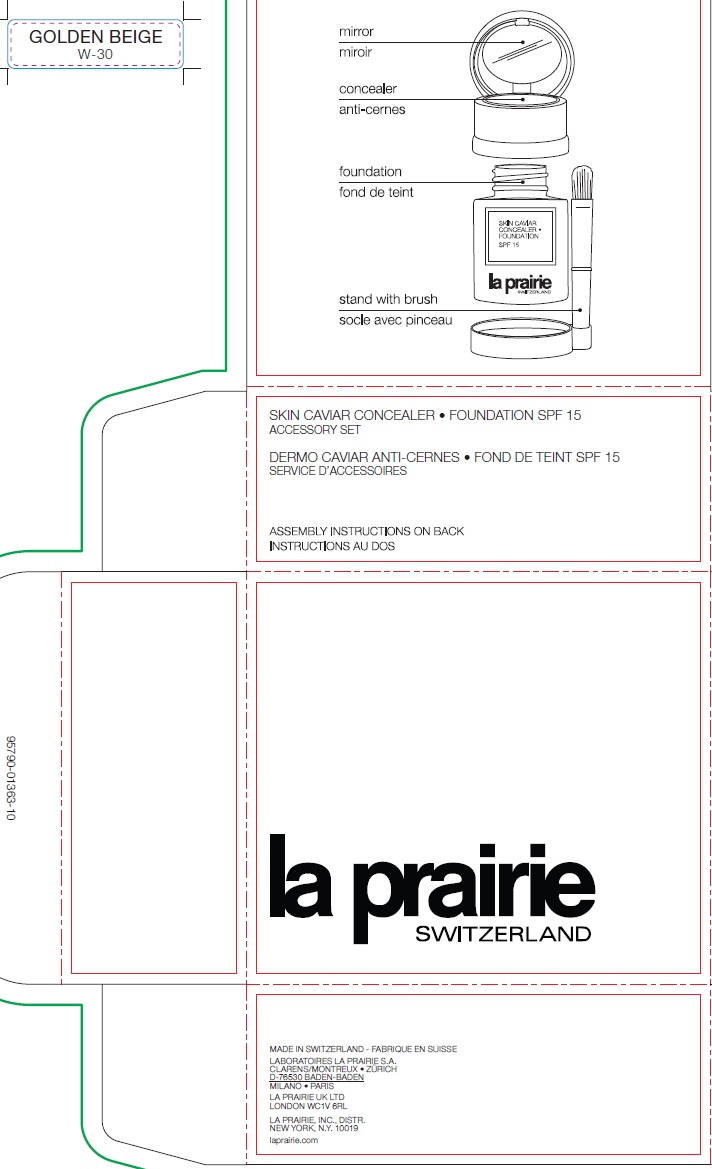
Warnings
Skin Cancer/Skin Aging Alert: Spending time in the sun increases your risk of skin cancer and early skin aging. This product has been shown only to prevent sunburn, not skin cancer or early skin aging.
For external use only
Directions
• apply daily after cleansing and toning
• smooth over face & throat
• apply liberally 15 minutes before sun exposure
• children under 6 months of age: Ask a doctor
• reapply at least every 2 hours
• use a water resistant sunscreen if swimming or sweating
Inactive Ingredients:
Water (Aqua), Cyclopentasiloxane, Phenyl Trimethicone, Butylene Glycol, Isononyl Isononanoate, PEG/PPG-18/18 Dimethicone, Polysilicone-11, Cyclohexasiloxane, Ethylhexyl Hydroxystearate, Diisopropyl Dimer Dilinoleate, Glycoproteins*, Panax Ginseng (Asian Ginseng) Root Extract*, Equisetum Arvense (Horsetail) Extract*, Palmitoyl Hexapeptide-14, C18-36 Acid Triglyceride, Caviar Extract, Haematococcus Pluvialis (Algae) Extract, Glycyrrhiza Glabra (Licorice) Root Extract, Lactobacillus Ferment, Tocopheryl Acetate, Glycine Soja (Soybean) Oil, Hydrolyzed Malt Extract, Chondrus Crispus (Carrageenan) Extract, Panthenol, Hydrolyzed Wheat Protein, Arnica Montana (Arnica) Flower Extract, Lactic Acid, Tocopherol, Glycerin, Ceramide NP, Sodium Hydroxide, Malic Acid, Biotin, Glycine, Arginine, Methionine, Disodium Stearoyl Glutamate, Petrolatum, Dimethicone, Tocopheryl Linoleate/Oleate, Ethylhexylglycerin, Propylene Glycol, Saccharomyces Cerevisiae Extract, Polymethyl Methacrylate, Lauryl Methacrylate/Glycol Dimethacrylate Crosspolymer, Aluminum Hydroxide, BHT, Trihydroxystearin, Polyglucuronic Acid, Ethoxydiglycol, Sodium Chloride, Fragrance (Parfum), Benzyl Salicylate, Linalool, Limonene, Alpha-Isomethyl Ionone, Geraniol, Citronellol, Pyruvic Acid, Methylparaben, Propylparaben, Phenoxyethanol, Ethylparaben May Contain [+/- : Titanium Dioxide (CI 77891), Iron Oxides (CI 77491), Iron Oxides (CI 77492), Iron Oxides (CI 77499)] i76
* New patent pending for La Prairie’s exclusive Cellular Complex in addition to US Patent 5,840,309