Uses
To reduce fever and for the temporary relief of minor aches and pains due to:
- •
- headache
- •
- muscular aches
- •
- backache
- •
- minor pain of arthritis
- •
- the common cold
- •
- toothache
- •
- premenstrual and menstrual cramps.
Temporarily reduces fever.
Warnings
Liver warning
This product contains acetaminophen. Severe liver damage may occur if
- •
- adult takes more than 6 doses in 24 hours, which is the maximum daily amount
- •
- child takes more than 5 doses in 24 hours, which is the maximum daily amount taken with other drugs containing acetaminophen
- •
- adult has 3 or more alcoholic drinks everyday while using this product.
Sore throat warning:
If sore throat is severe, persists for more than 2 days, is accompanied or followed by fever, headache, rash, nausea, or vomiting, consult a doctor promptly.
Do not use
- with any other drug containing acetaminophen (prescription or non-prescription). If you are not sure whether a drug contains acetaminophen, ask a doctor or pharmacist.
- if your child is allergic to acetaminophen or any of the inactive ingredients in this product
Ask a doctor or pharmacist before use if your child is taking the blood thinning drug warfarin
When using this product: Do not exceed recommended dose
Stop use and ask a doctor if:
- •
- Pain gets worse or lasts more than 5 days
- •
- fever gets worse or lasts more than 3 days
- •
- new symptoms occur.
- •
- redness or swelling is present.
These could be signs of a serious condition.
Keep out of reach of children.
Overdose Warning:
taking more than the recommended dose (overdose) may cause liver damage.
In case of overdose get medical help or contact a Poison Control Center right away. Quick medical attention is critical for adults/children even if you do not notice any signs or symptoms.
Directions
- •
- shake well before using
- •
- find the right dose on chart below, if possible, use weight to dose; otherwise use age
- •
- dosage may be repeated every 4 hours, or as directed by your doctor
- •
- do not use more than 5 doses in 24 hours
- •
- do not use more than 5 days unless directed by a doctor.
- •
- find right dose on chart below, If possible, use weight to dose; otherwise, use age.
| Weight (lbs.) | Age (years) | dosage-teaspoonful (tsp.) |
| under 24 | under 2 | consult Physician |
| 24 to 35 | 2 to 3 | 1 tsp. (5 mL) |
| 36 to 47 | 4 to 5 | 1 1/2 tsp. (7.5 mL) |
| 48 to 59 | 6 to 8 | 2 tsp. (10 mL) |
| 60 to 71 | 9 to 10 | 2 1/2 tsp. (12.5 mL) |
|
72 to 95 |
11 |
3 tsp. (15 mL) |
Other information
- •
- Store at room temperature 15°-30° C (59°-86°F)
- •
- Protect from Freezing.
- •
- Protect from Light.
TAMPER EVIDENT: DO NOT USE IF BREAKAWAY BAND ON CAP IS BROKEN OR MISSING.
Inactive Ingredients:
Grape Flavor, Citric Acid, Glycerin, Polyethylene Glycol 400, Purified Water, Sodium Citrate, Sodium Saccharin, Sorbitol Solution 70% & Sodium Benzoate.
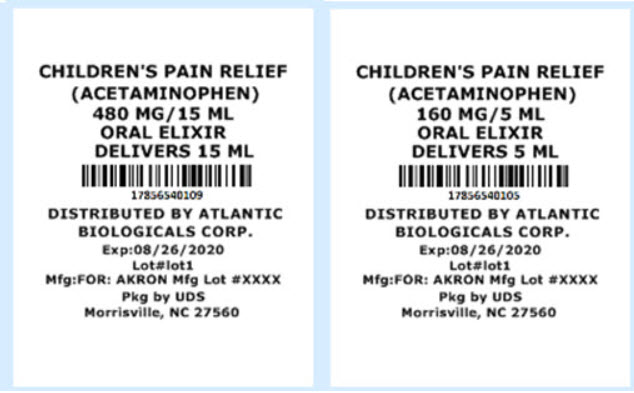
How Supplied:
17856-5401-01 ACETAMINOPHEN - 48MG/1.5ML SYRINGE 120 UD
17856-5401-02 ACETAMINOPHEN - 64MG/2ML SYRINGE 120 UD
17856-5401-03 ACETAMINOPHEN - 96MG/3ML SYIRNGE 120 UD
17856-5401-04 ACETAMINOPHEN - 3.75 ML SYRINGE 48 ct UD
17856-5401-05 ACETAMINOPHEN - 5 ML CUP 72 ct UD
17856-5401-06 ACETAMINOPHEN - 192MG/6ML SYRINGE 48 ct UD
17856-5401-07 ACETAMINOPHEN - 240MG/7.5ML SYRINGE 48 ct UD
17856-5401-08 ACETAMINOPHEN - 325MG/10.15 ML CUP 72 ct UD
17856-5401-09 ACETAMINOPHEN - 480MG/15ML 50 CUP ct UD
Questions or Comments?
Call (877) 225-6999 Monday - Friday 9AM-5PM EST
Manufactured for
Akron Pharma, Inc.,
Fairfeld, NJ - 07004
Manufactured In USA
* This product is not manufactured or distributed by McNeil Consumer Healthcare, owner of the registered trademark Tylenol Elixir
Distributed by:
ATLANTIC BIOLOGICALS CORP.
Miami, FL 33179