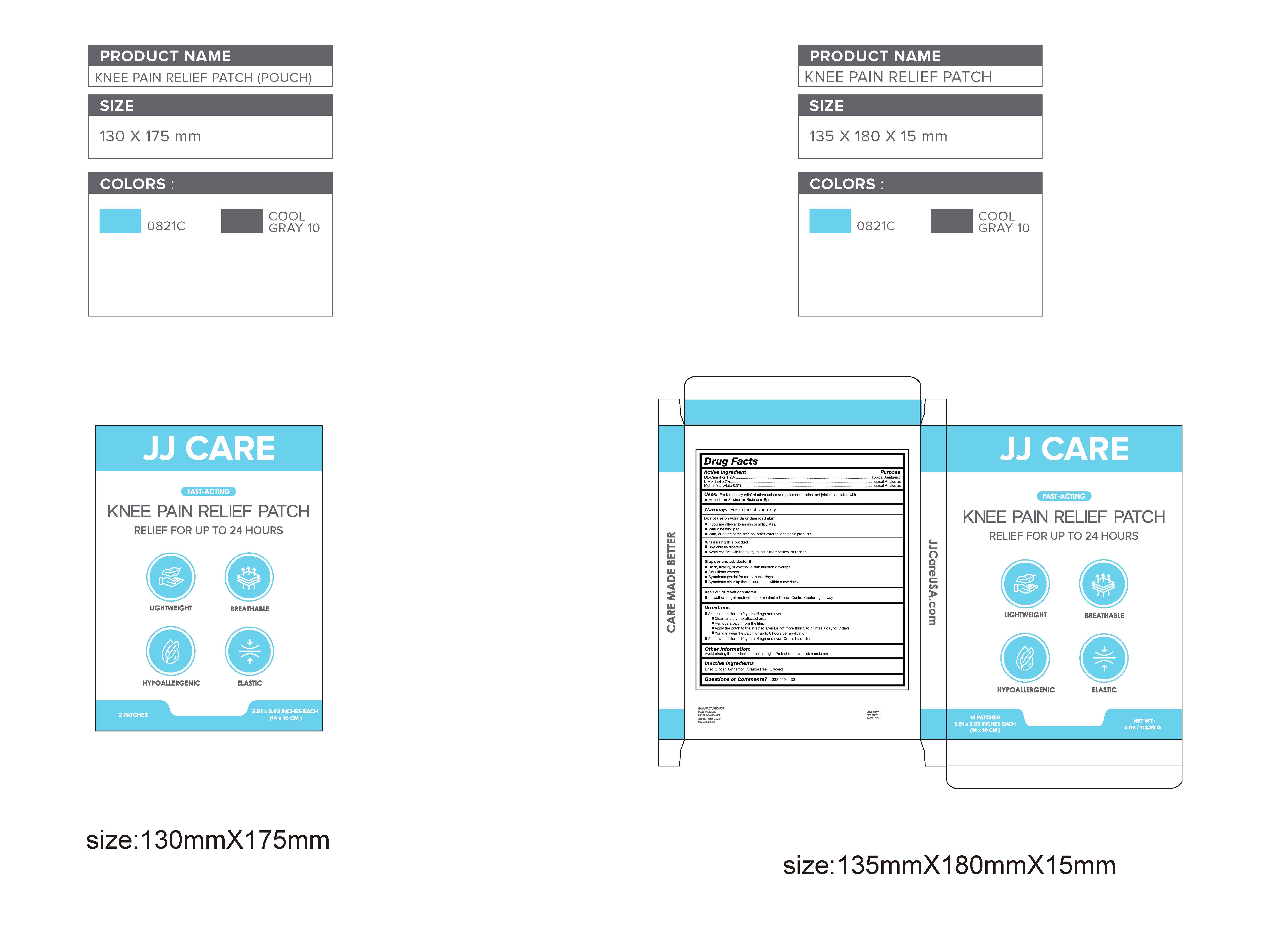
DL-Camphor 1.2% / Topical Analgesic
L-Menthol 5.7% / Topical Analgesic
Methyl Salicylate6.3% / Topical Analgesic
For temporary relief of minor aches and pains of muscles and joints associated with:Arthritis,Strains,Bruises,Sprains
Do not use on wounds or damaged skin
If you are allergic to aspirin or salicylates.
With a heating pad.
With, or at the same time as, other external analgesic products.
When using this product:
Use only as directed.
Avoid contact with the eyes, mucous membranes, or rashes.
Rash, itching, or excessive skin irritation develops.
Conditions worsen.
Symptoms persist for more than 7 days.
Symptoms clear up then occur again within a few days.
Keep out of reach of children.If swallowed, get medical help or contact a Poison Control Center right away.