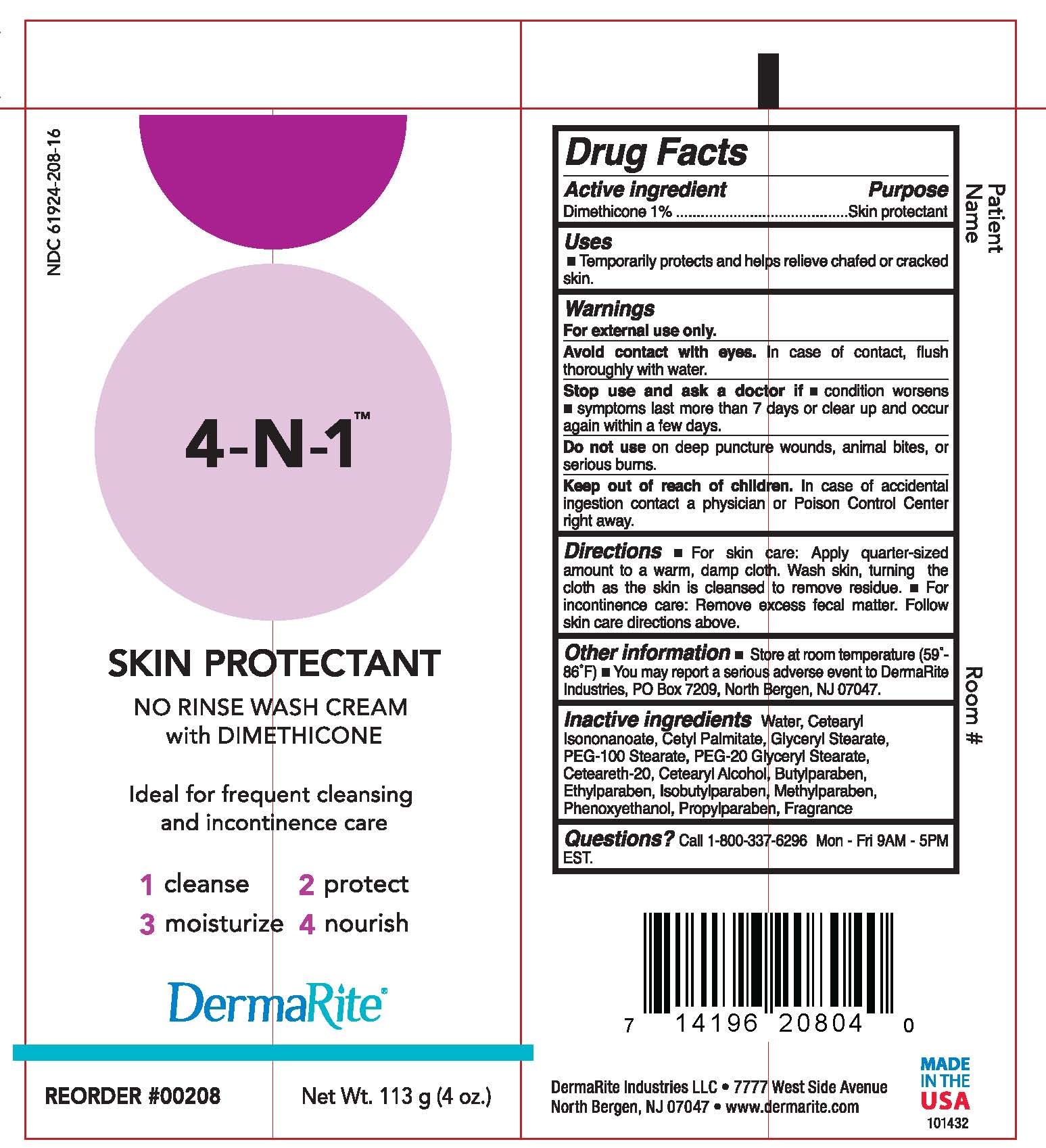
4-N-1 WASH- otc skin protectant drug product cream
DermaRite Industries, LLC
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Active Ingredients:
Dimethicone 1%
Uses:
- A gentle alternative to soap and water.
- Cleans, restores, nourishes, and protects even most delicate skin.
- Ideal for frequent cleansing and incontinence care.
Warnings:
-
For external use only.
-
Avoid contact with eyes. In case of contact, flush thoroughly with water.
-
Stop use and ask a doctor if – condition worsens or does not improve within 7 days; symptoms clear up and occur again within a few days.
-
Do not use on deep puncture wounds, animal bites, or serious burns.
Warnings:
-
Keep out of reach of children. In case of accidental ingestion contact a physician or Poison Control Center right away.
Directions:
-
For skin care: Apply quarter-sized amount to warm, damp cloth. Wash skin, turning the cloth as the skin is cleansed to remove residue.
-
For incontinence care: Remove excess fecal matter. Follow skin care directions above
Other Information:
Store at room temperature (59°-86°F)
Ingredients:
Butylparaben, Ceteareth-20, Cetearyl Alcohol, Cetearyl Isononanoate, Cetyl Palmitate, Ehtylparaben, Fragrance, Glyceryl Stearate, Isobutylparaben, Methyl paraben, PEG-100 Stearate, PEG-20 Glyceryl Stearate, Phenoxyethanol, Propylparaben, Water
4-N-1 Wash Cream Package Label Principal Display Panel