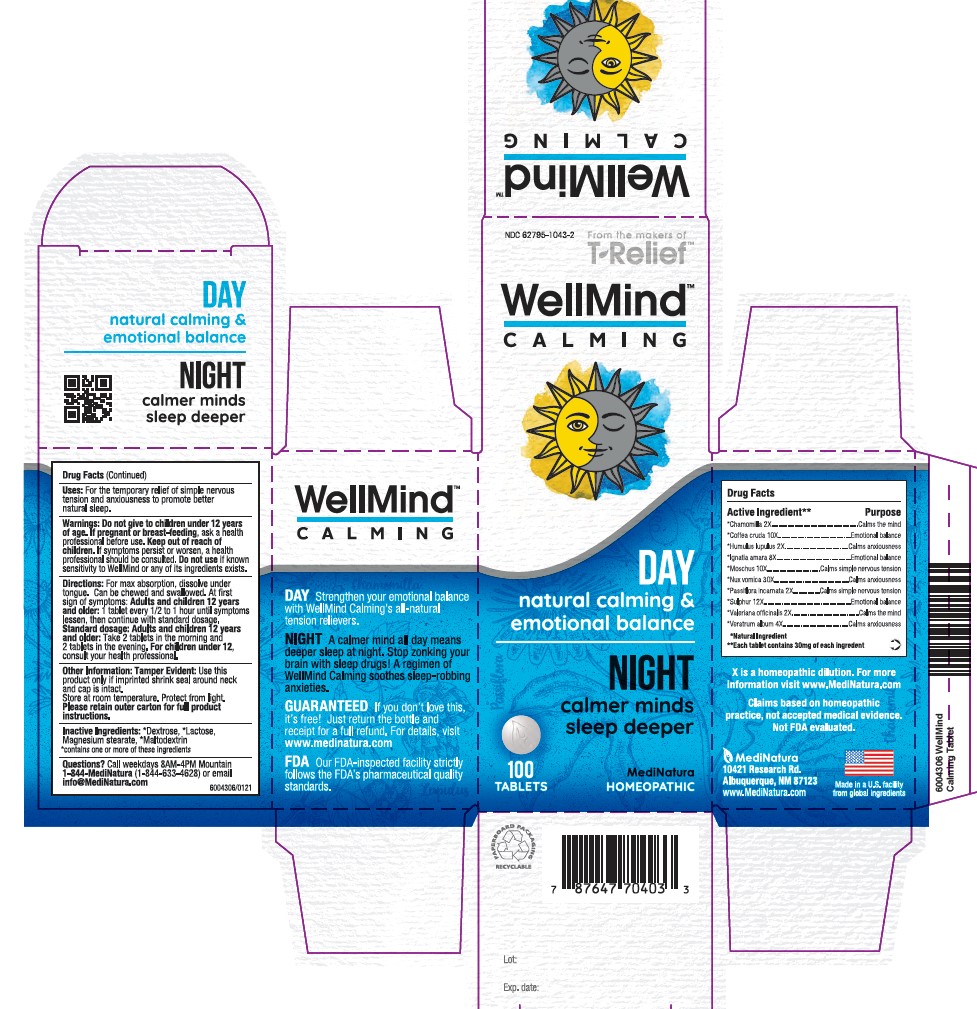
Uses
For the temporary relief of simple nervous tension and anxiousness to promote better natural sleep.
WARNINGS
Warnings: Do not give to children under 12 years of age.If pregnant or breast-feeding, ask a health professional before use. KEEP OUTOF REACH OF CHILDREN. If symptoms persist or worsen, a health professional should be consulted. Do not use if known sensitivity to WellMind or any of it's ingredients exists.
DIRECTIONS
For max absorption, dissolve under tongue. Can be chewed and swallowed. At first sign of symptoms: Adults and children 12 years and older: 1 tablet every ½ to 1 hour until symptoms lessen, then continue with standard dosage.Standard dosage: Adults and children 12 years and older: take 2 tablets in the moning and 2 tablets in the evening. For children under 12 years, consult your health professional.
ACTIVE INGREDIENTS
Chamomilla 2X, Coffea cruda 10X, Humulus lupulus 2X, Ignatia amara 8X, Moschus 10X,
Nux-vomoica 30X, Passiflora incarnata 2X, Sulphur 12X, Valeriana officinalis 2X, Veratrum album 4X