Purpose
Lidocaine (4%)..................Topical Anesthetic
Menthol (1%)....................Topical Anesthetic
Warnings
For external use only
Do not use
- more than one patch at a time
- on wounds, cuts, damaged, broken and/or irritated skin
- over raw surfaces or blistered areas
- at the same time as other topical analgesics
- if allergic to menthol, lidocaine or any other inactive ingredients
- if package is damaged or opened
When using this product
- use only as directed. Read and follow all directions and warnings on this label
- avoid contact with eyes or mucous membranes
- do not bandage tightly or apply local heat (such as heating pads) to the area of use
Directions
Adults and children over 12 years of age
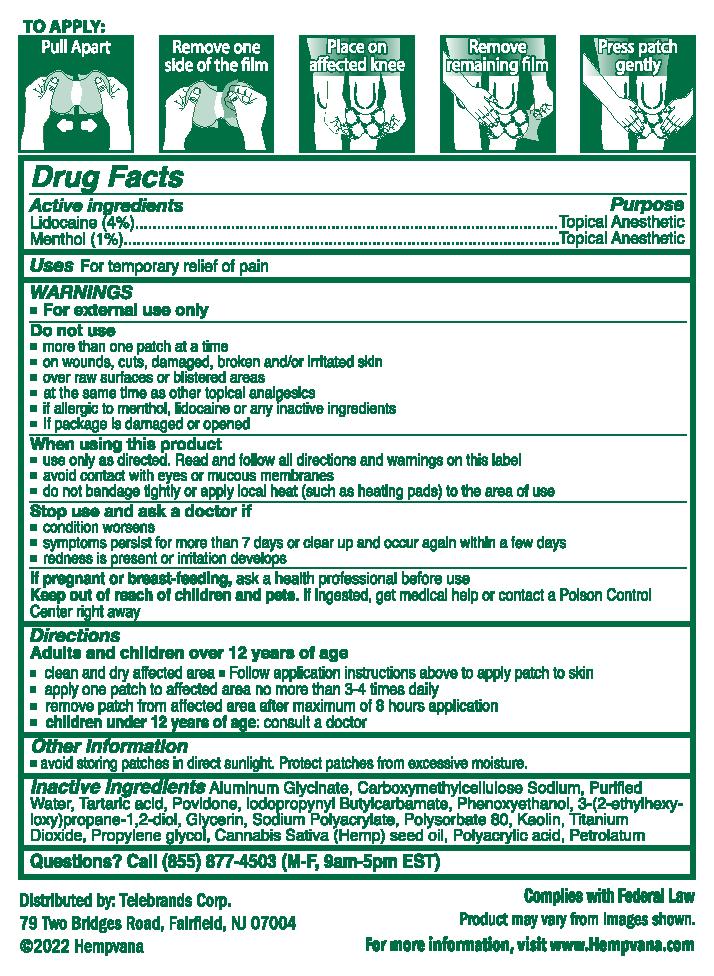
- clean and dry affected area
- follow application instructions above to apply patch to skin
- apply one patch to affected area no more than 3-4 times daily
- remove patch from affected area after maximum of 8 hours application
- children under 12 years of age: consult a doctor
Other information
- avoid storing patches in direct sunlight. Protect patches from excessive moisture.
Inactive ingredients
Aluminum Glycinate, Carboxymethylcellulose Sodium, Purified Water, Tartaric acid, Povidone, Iodopropynyl Butylcarbamate, Phenoxyethanol, 3-(2-ethylhexyloxy)propane-1,2-diol, Glycerin, Sodium Polyacrylate, Polysorbate 80, Kaolin, Titanium Dioxide, Propylene Glycol, Cannabis Sativa (Hemp) Seed Oil, Polyacrylic acid, Petrolatum
#1 Hemp Pain Relief Brand in America 1
1AC NIELSEN 2021 Food Drug Mass POS
Pain Relief for Your Knees
Hempvana
Kneebird
Ultra Strength Pain RElief Patches
Bends & Flexes as you move!
As Seen on TV
Not actual size
For Even More Moisturization
Lidocaine 4%
Menthol 1%
For Pain
*Compared to original Hempvana Knee Bird
12 Pourches
Patches 3.93"x6.10"
(10cm x 15.49cm) each