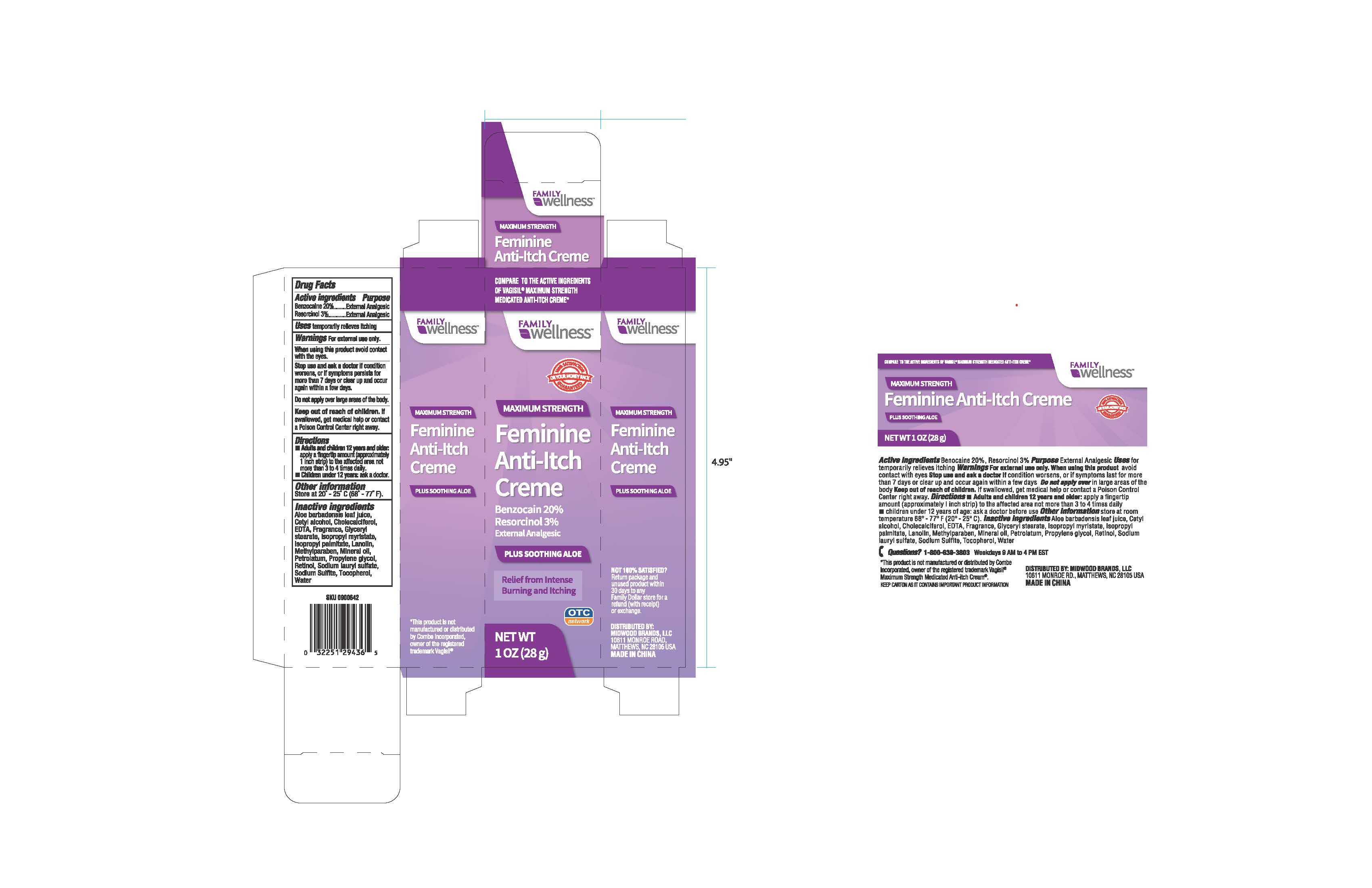
. Warnings For external use only
avoid contact When using this product
with the eyes.
if condition Stop use and ask a doctor
worsens, or if symptoms persists for
more than 7 days or clear up and occur
again within a few days.
over large areas of the body. Do not apply
. If Keep out of reach of children
swallowed, get medical help or contact
a Poison Control Center right away.
Directions
• Adults and children 12 years and older:
apply a fingertip amount (approximately
1 inch strip) to the affected area not
more than 3 to 4 times daily.
•: ask a doctor Children udner 12 years