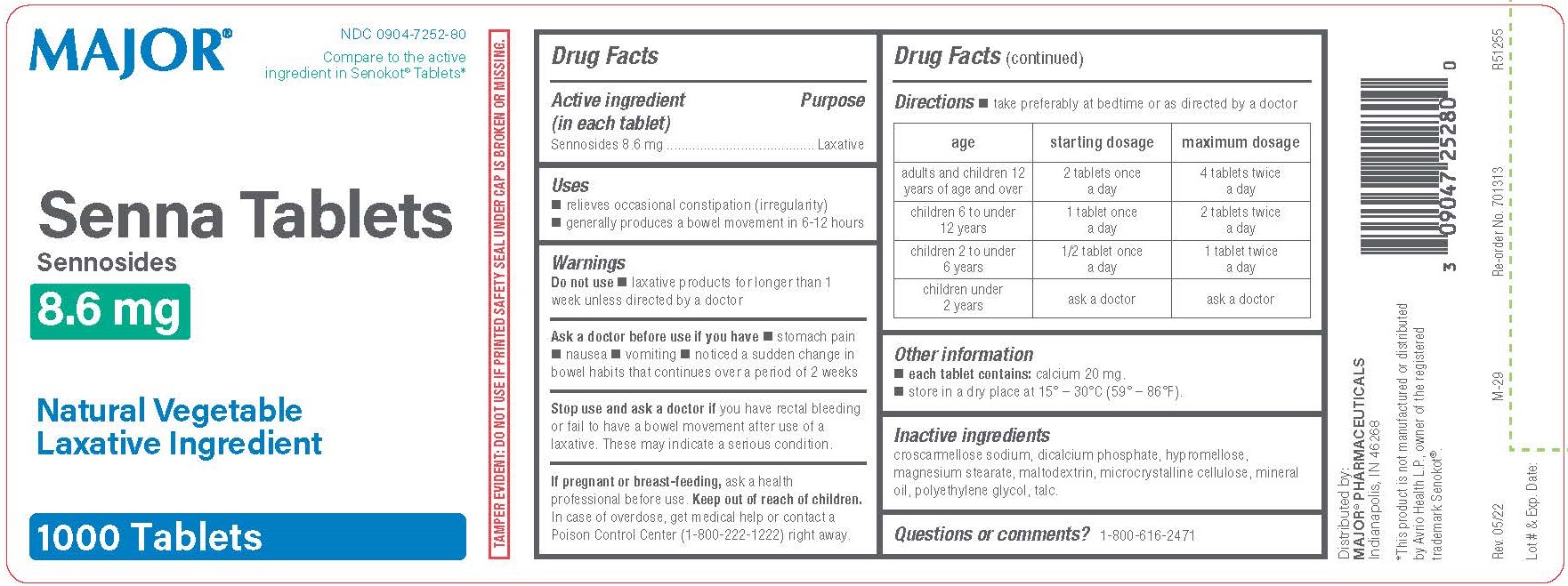
SENNOSIDES- sennosides tablet, film coated
Major Pharmaceuticals
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Drug Facts
Active ingredient (in each tablet)
Sennosides 8.6 mg
Uses
relieves occasional constipation (irregularity)
generally produces a bowel movement in 6-12 hours
laxative products for longer than 1 week unless directed by a doctor
Ask a doctor before use if you have
stomach pain
nausea
vomiting
noticed a sudden change in bowel habits that continues over a period of 2 weeks
Stop use and ask a doctor if you have rectal bleeding or fail to have a bowel movement after use of a laxative. These may indicate a serious condition.
If pregnant or breast-feeding, ask a health professional before use.
Keep out of reach of children.
In case of overdose, get medical help or contact a Poison Control Center (1-800-222-1222) right away.
Directions
take preferably at bedtime or as directed by a doctor
| age | starting dosage | maximum dosage |
adults and children 12
years of age and over
| 2 tablets once
a day
| 4 tablets twice
a day
|
children 6 to under
12 years
| 1 tablet once
a day
| 2 tablets twice
a day
|
children 2 to under
6 years
| 1/2 tablet once
a day
| 1 tablet twice
a day
|
children under
2 years
| ask a doctor | ask a doctor |
Other information
each tablet contains: calcium 20 mg.
store in a dry place at 15° – 30°C (59° – 86°F).
croscarmellose sodium, dicalcium phosphate, hypromellose, magnesium stearate, maltodextrin, microcrystalline cellulose, mineral oil, polyethylene glycol, talc.
Questions or comments?
1-800-616-2471
TAMPER EVIDENT: DO NOT USE IF PRINTED SAFETY SEAL UNDER CAP IS BROKEN OR MISSING.
Distributed by:
MAJOR® PHARMACEUTICALS
Indianapolis, IN 46268
*This product is not manufactured or distributed by Avrio Health L.P., owner of the registered trademark Senokot®.
Major
NDC 0904-7252-80
Compare to the active ingredient in Senokot® Tablets*
Senna Tablets
Sennosides
8.6 mg
Natural Vegetable Laxative Ingredient
1000 Tablets