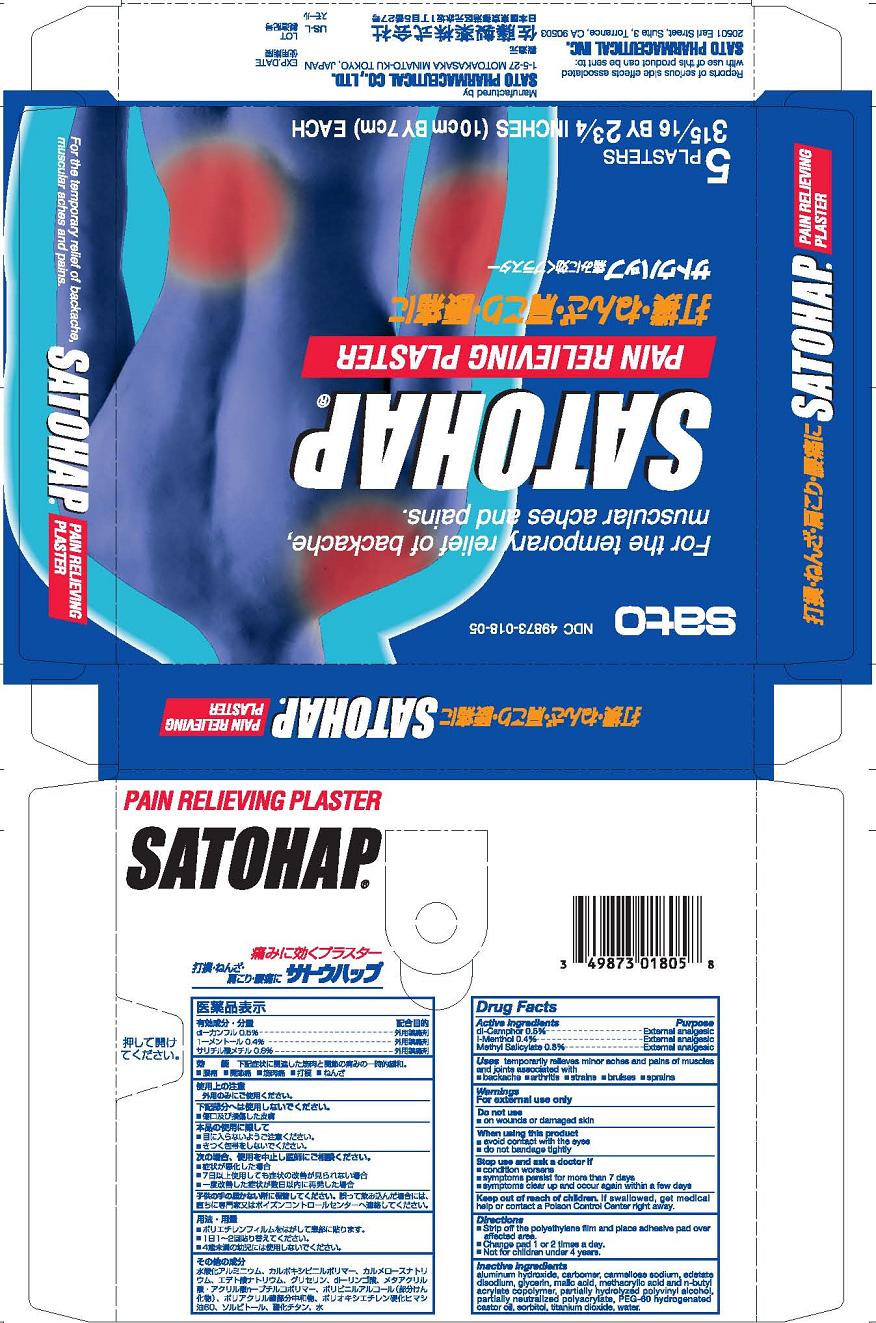
Purpose
dl-Camphor External analgesic
l-Menthol External analgesic
Methyl Salicylate External analgesic
Uses temporary relieves minor aches and pains of muscles and joints associated with
■ simple backache ■ arthritis ■ strains ■ bruises ■ sprains
Warnings
For external use only
Directions
■ Strip off the polyethylene film and place adhesive pad over affected area.
■ Change pad 1 or 2 times a day
■ Not for children under 4 years.
Inactive ingredients
aluminum hydroxide gel, carbomer, carmellose sodium, edetate disodium, glycerin, malic acid, methacrylic acid and n-butyl acrylate copolymer, partially hydrolyzed polyvinyl alcohol, partially neutralized polyacrylate, PEG 60 hydrogenated castor oil, sorbitol, titanium dioxide, water.