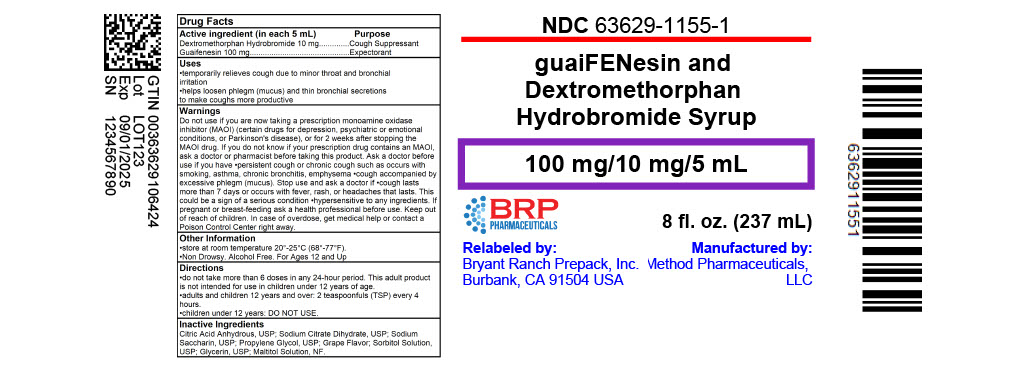
Uses
- temporarily relieves cough due to minor throat and bronchial
irritation - helps loosen phlegm (mucus) and thin bronchial secretions
to make coughs more productive
Warnings
Do not use if you are now taking a prescription
monoamine oxidase inhibitor (MAOI) (certain drugs
for depression, psychiatric or emotional conditions,
or Parkinson's disease), or for 2 weeks after
stopping the MAOI drug. If you do not know if
your prescription drug contains an MAOI, ask a
doctor or pharmacist before taking this product.
Ask a doctor before use if you have
- persistent cough or chronic cough such as occurs with smoking, asthma, chronic bronchitis, emphysema
- cough accompanied by excessive phlegm (mucus)
Stop use and ask a doctor if
- cough lasts more than 7 days or occurs with fever, rash, or
headaches that lasts. This could be a sign of a serious condition - hypersensitive to any ingredients
Keep out of reach of children.
In case of overdose, get medical help or contact a Poison Control Center right away.
Directions
- do not take more than 6 doses in any 24-hour period. This adult product is not intended for use in children under 12 years of age
| adults and children 12 years and over | 2 teaspoonfuls (TSP) every 4 hours |
| children under 12 years | DO NOT USE |
Inactive ingredients
Citric Acid Anhydrous, USP; Sodium Citrate Dihydrate, USP; Sodium Saccharin, USP; Propylene Glycol, USP; Grape Flavor; Sorbitol Solution, USP; Glycerin, USP; Maltitol Solution, NF