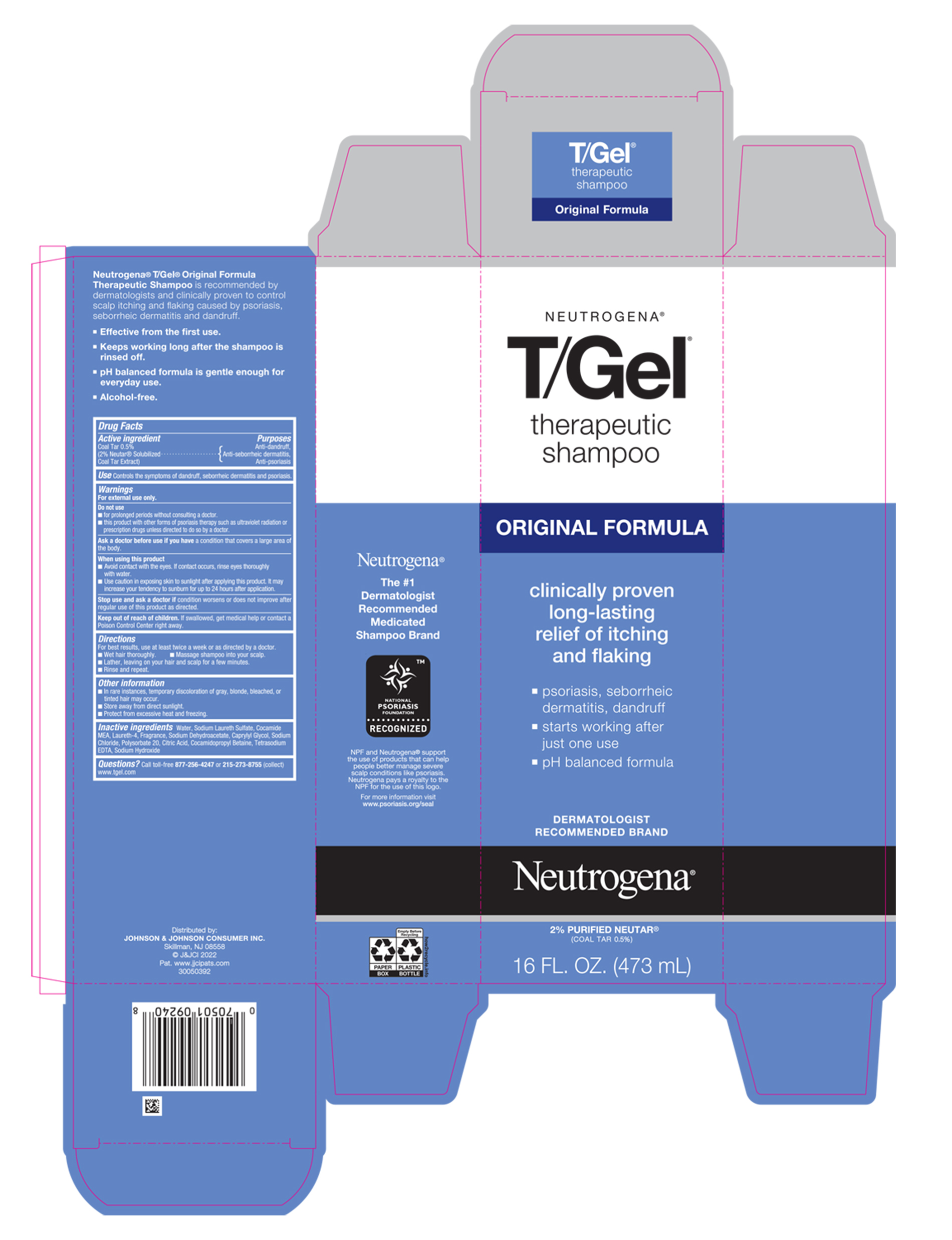
Warnings
For external use only.
Do not use
- for prolonged periods without consulting a doctor.
- this product with other forms of psoriasis therapy such as ultraviolet radiation or prescription drugs unless directed to do so by a doctor.
When using this product
- Avoid contact with the eyes. If contact occurs, rinse eyes thoroughly with water.
- Use caution in exposing skin to sunlight after applying this product. It may increase your tendency to sunburn for up to 24 hours after application.
Directions
- For best results, use at least twice a week or as directed by a doctor.
- Wet hair thoroughly.
- Massage shampoo into your scalp.
- Lather, leaving on your hair and scalp for a few minutes.
- Rinse and repeat.
Other information
- In rare instances, temporary discoloration of gray, blonde, bleached, or tinted hair may occur.
- Store away from direct sunlight.
- Protect from excessive heat and freezing.
Inactive ingredients
Water, Sodium Laureth Sulfate, Cocamide MEA, Laureth-4, Fragrance, Sodium Dehydroacetate, Caprylyl Glycol, Sodium Chloride, Polysorbate 20, Citric Acid, Cocamidopropyl Betaine, Tetrasodium , Tetrasodium EDTA, Sodium Hydroxide
PRINCIPAL DISPLAY PANEL - 473 mL Bottle Carton
NEUTROGENA®
T/GEL®
therapeutic
shampoo
ORIGINAL FORMULA
clinically proven
long-lasting
relief of itching
and flaking
psoriasis, seborrheic
dermatitis, dandruff
starts working after
just one use
pH balanced formula
DERMATOLOGIST
RECOMMENDED BRAND
Neutrogena®
2% PURIFIED NEUTAR®
(COAL TAR 0.5%)
16 FL. OZ. (473 mL)