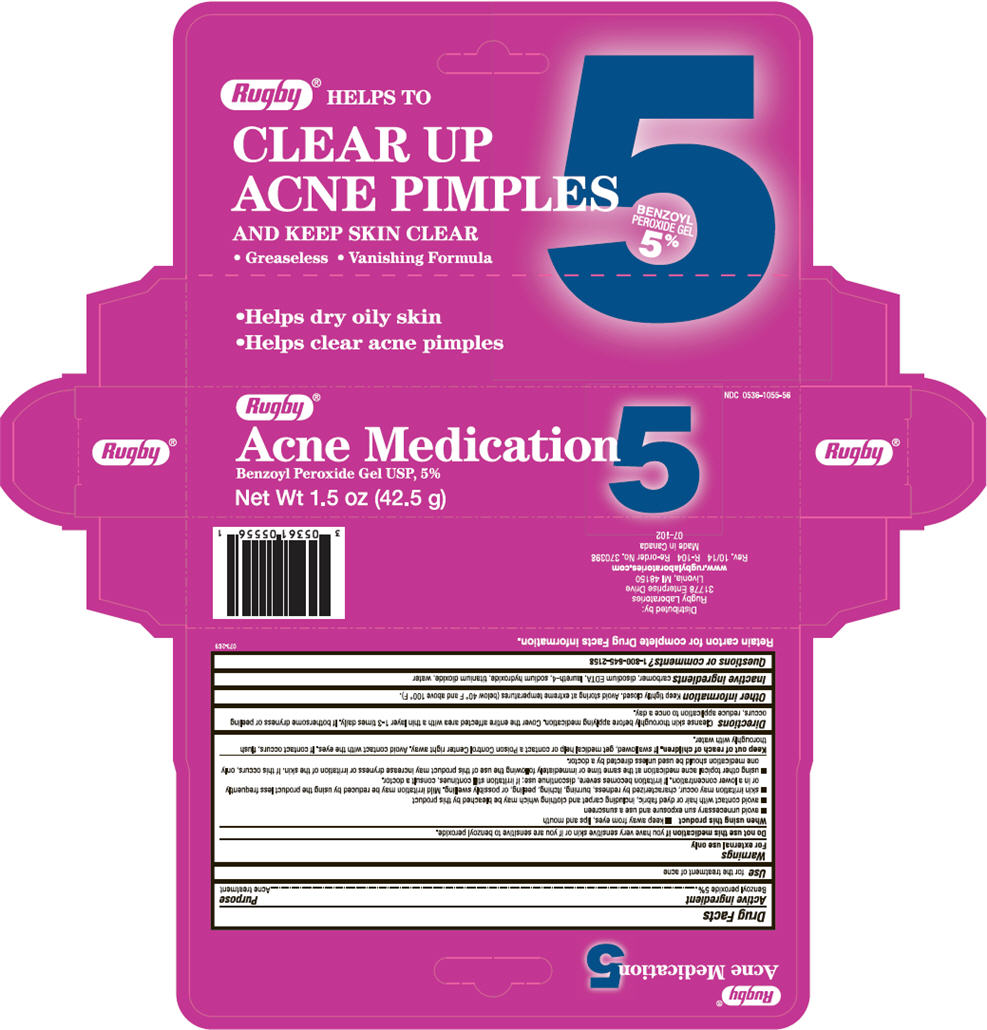
Warnings
For external use only
Do not use this medication if you have very sensitive skin or if you are sensitive to benzoyl peroxide.
When using this product
n keep away from eyes, lips and mouth
- avoid unnecessary sun exposure and use a sunscreen
- avoid contact with hair or dyed fabric, including carpet and clothing which may be bleached by this product
- skin irritation may occur, characterized by redness, burning, itching, peeling, or possibly swelling. Mild irritation may be reduced by using the product less frequently or in a lower concentration. If irritation becomes severe, discontinue use; if irritation still continues, consult a doctor.
- using other topical acne medication at the same time or immediately following the use of this product may increase dryness or irritation of the skin. If this occurs, only one medication should be used unless directed by a doctor.
Directions
Cleanse skin thoroughly before applying medication. Cover the entire affected area with a thin layer 1-3 times daily. If bothersome dryness or peeling occurs, reduce application to once a day.