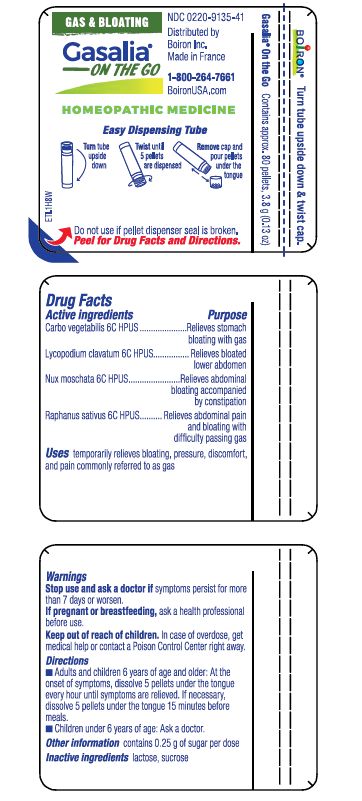
Active ingredients** (in each pellet)
Carbo vegetabilis 6C HPUS (0.11 mg)
Lycopodium clavatum 6C HPUS (0.11 mg)
Nux Moschata 6C HPUS (0.11 mg)
Raphanus sativus 6C HPUS (0.11 mg)
The letters "HPUS" indicate that the components in this product are officially monographed in the Homeopathic Pharmacopoeia of the United States.
Purpose*
Carbo Vegetabilis 6C HPUS (0.11 mg) ... Relieves stomach bloating with gas
Lycopodium clavatum 6C HPUS (0.11 mg) ... Relieves bloated lower abdomen
Nux moschata 6C HPUS (0.11 mg) ... Relieves abdominal bloating accompanied by constipation
Raphanus sativus 6C HPUS (0.11 mg) ... Relieves abdominal pain and bloating with difficulty passing gas
Keep out of reach of children. In case of overdose, get medical help or contact a Poison Control Center right away.
- Adults and children 6 years of age and older: At the onset of symptoms, dissolve 5 pellets under the tongue every hour until symptoms are relieved. If necessary, dissolve 5 pellets under the tongue 15 minutes before meals.
- Children under 6 years of age: Ask a doctor.
- do not use if glue carton end flaps are open if pellet dispenser seal is broken
- contains 0.25 g of sugar per pose
Multi-Symptom
Gas & Bloating Relief*
Plant-Powered Relief
Meltaway Pellets
2 PORTABLE TUBES
APPROX. 80 PELLETS EACH
TOTAL 160 PELLETS
Contains approx. 80 pellets, 3.8 g (0.13 oz)
Turn tube upside down
Twist until 5 pellets are dispensed
Remove cap and pour pellets under the tongue
Do not use if pellet dispenser seal is broken.
*CLAIMS BASED ON TRADITIONAL HOMEOPATHIC PRACTICE NOT ACCEPTED MEDICAL EVIDENCE. NOT FDA EVALUATED.
**C,K,CK, and X are homeopathic dilutions: see BoironUSA.com/info for details.