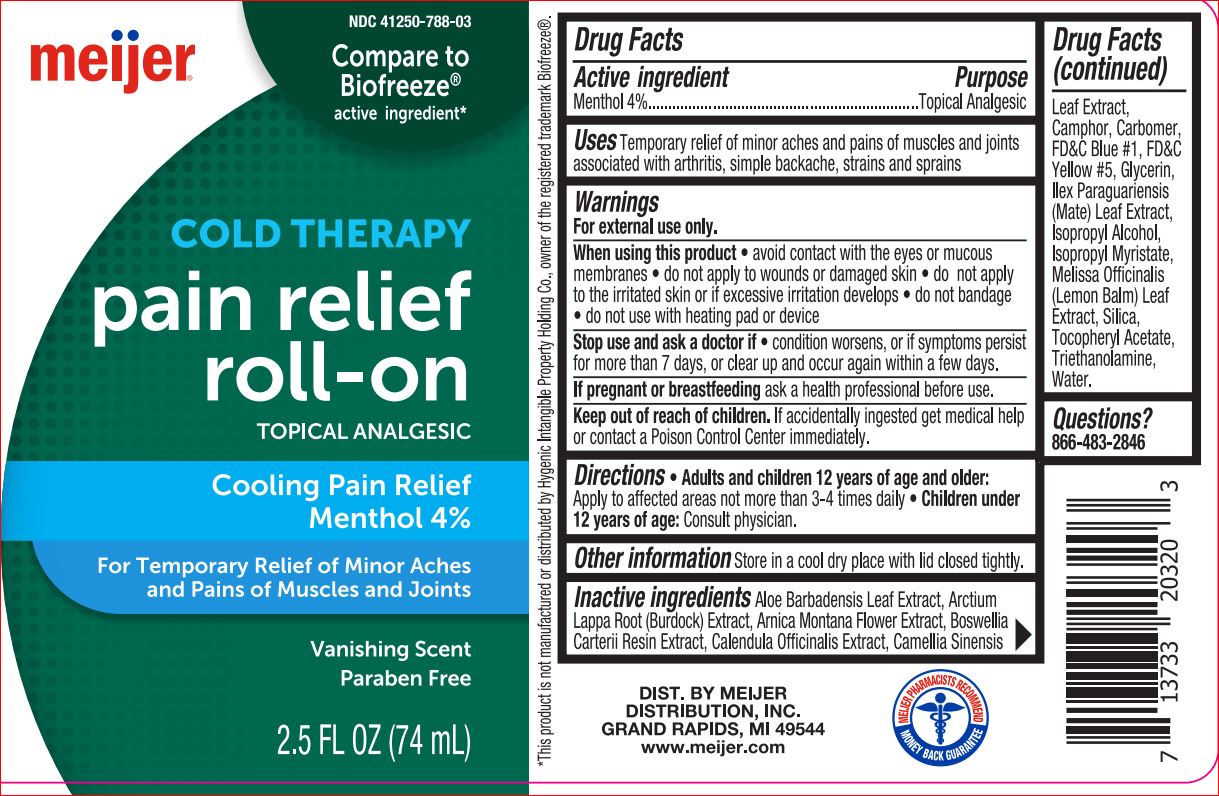
Active ingredient Purpose
Menthol 4%..........................................................Topical Analgesic
Uses
Temporary relief of minor aches and pains of muscles and joints
associated with arthritis, simple backache, strains and sprains
Warnings
For external use only.
When using this product • avoid contact with the eyes or mucous
membranes • do not apply to wounds or damaged skin • do not
apply to the irritated skin or if excessive irritation develops • do not
bandage • do not use with heating pad or device
Stop use and ask a doctor if • condition worsens, or if symptoms
persist for more than 7 days, or clear up and occur again within a
few days.
If pregnant or breastfeeding ask a health professional before use
Keep out of reach of children. If accidentally ingested get
medical help or contact a Poison Control Center immediately.
Directions
• Adults and children 12 years of age and older: Apply on to the
affected areas not more than 3-4 times daily.
• Children under 12 years of age: Consult physician
Inactive ingredients
Aloe Barbadensis Leaf Extract, Arctium Lappa Root (Burdock)
Extract, Arnica Montana Flower Extract, Boswellia Carterii Resin
Extract, Calendula Officinalis Extract, Camellia Sinensis Leaf Extract,
Camphor, Carbomer, FD&C Blue #1, FD&C Yellow #5, Glycerin, Ilex
Paraguariensis (Mate) Leaf Extract, Isopropyl Alcohol, Isopropyl
Myristate, Melissa Officinalis (Lemon Balm) Leaf Extract, Silica,
Tocopheryl Acetate, Triethanolamine, Water.