DRUG FACTS
ACTIVE INGREDIENTS (per ml) PURPOSE
- Lidocaine USP - 3%
topical anesthetic
- Tetracaine USP - 2%
topical anesthetic

OTHER INGREDIENTS
Deionized water, caprylc/capric triglyceride,stearic acid, glycerin, glyceryl stearate, petrolatum, safflower oil, shea butter, polysorbate 60, stearyl alcohol, sweet almond oil, tocopheryl acetate, bht, propylene glycol, sodium carbonate, phenoxyethanol, methylparaben, polyparaben. May contain: FD&C Green #3, FD&C Yellow #5, FD&C Blue #1
DO NOT USE
- With occlusion
- If you have a known allergy or sensitivity to any ingredients
- If pregnant or breastfeeding
- On large areas of the body
- In large quantities. Apply only 1-2ml to desired area
- If you have a history of liver disease or impairment
- If safety seal is broken before first use
KEEP OUT OF
The eye and mouth. If accidental contact occurs, you may feel burning or stinging. Wash with water or eyewash immediately. If your eyes have pain, blurry vision, extreme sensitivity to light, or a feeling of sand in the eye, contact an eye care physician immediately.
WHEN USING THIS PRODUCT
You may not feel pain. Avoid sources of heat or injury.
You may have temporary rash, redness, swelling, or itching
Contact your physician promptly if you notice any unusual effects such as dizziness or drowsiness, difficulty breathing, trembling, chest pain, or irregular heartbeat.
Made in the USA for SofTap
550 N Canyons Pkwy
Livermore, CA 94551
www.softaps.com

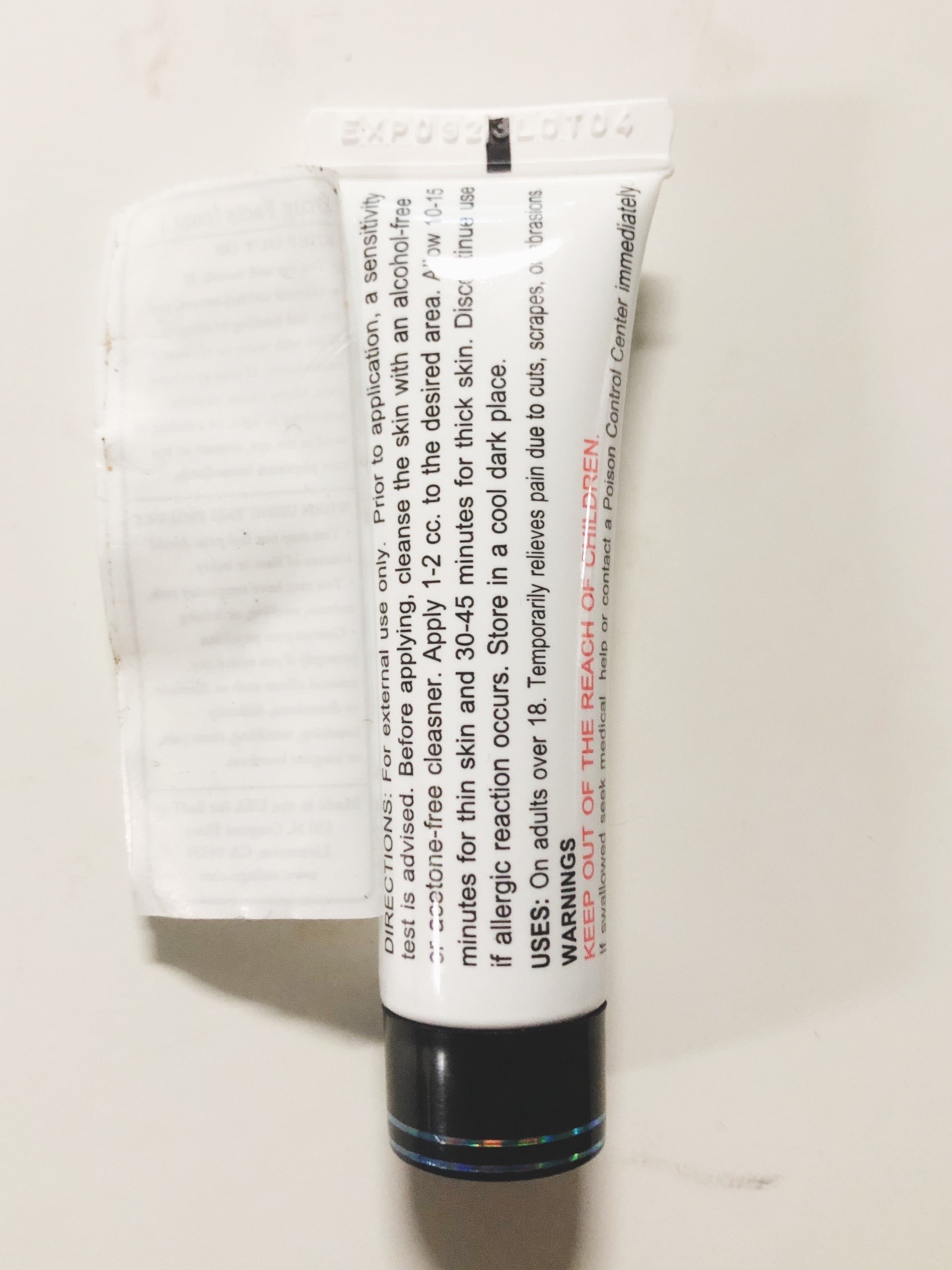
DIRECTIONS
For external use only. Prior to application, a sensitivity test is advised. Before applying, cleanse the skin with an alcohol-free or acetone-free cleanser. Apply 1-2 cc to the desired area for up to 6 times per day. Discontinue use if allergic reaction occurs. Store in a cool dark place (up to 80 degrees F) or refrigerate.