Uses
- temporarily relieves these symptoms due to hay fever or other upper respiratory allergies:
- runny nose
- sneezing
- itchy, watery eyes
- itching of the nose or throat
- temporarily relieves these symptoms due to the common cold:
- runny nose
- sneezing
Warnings
Do not use
- to make a child sleepy
- with any other product containing diphenhydramine, even one used on skin
Ask a doctor before use if you have
- a breathing problem such as emphysema or chronic bronchitis
- glaucoma
- trouble urinating due to an enlarged prostate gland
Directions
- take every 4 to 6 hours, or as directed by a doctor
- do not take more than 6 times in 24 hours
| adults and children 12 years and over | 1 to 2 tablets |
| children 6 to under 12 years | 1 tablet |
| children under 6 years | do not use |
Other information
- each tablet contains: calcium 15 mg
- store between 20-25°C (68-77°F). Protect from light.
- do not use if pouch is torn or damaged
Inactive ingredients
carnauba wax, croscarmellose sodium, D&C red no. 27 aluminum lake, dibasic calcium phosphate, hypromellose, magnesium stearate, microcrystalline cellulose, polyethylene glycol, polysorbate 80, titanium dioxide
Repackaged and distributed by:
Lil' Drug Store Products, Inc., 1201 Continental Place NE, Cedar Rapids, IA 52402
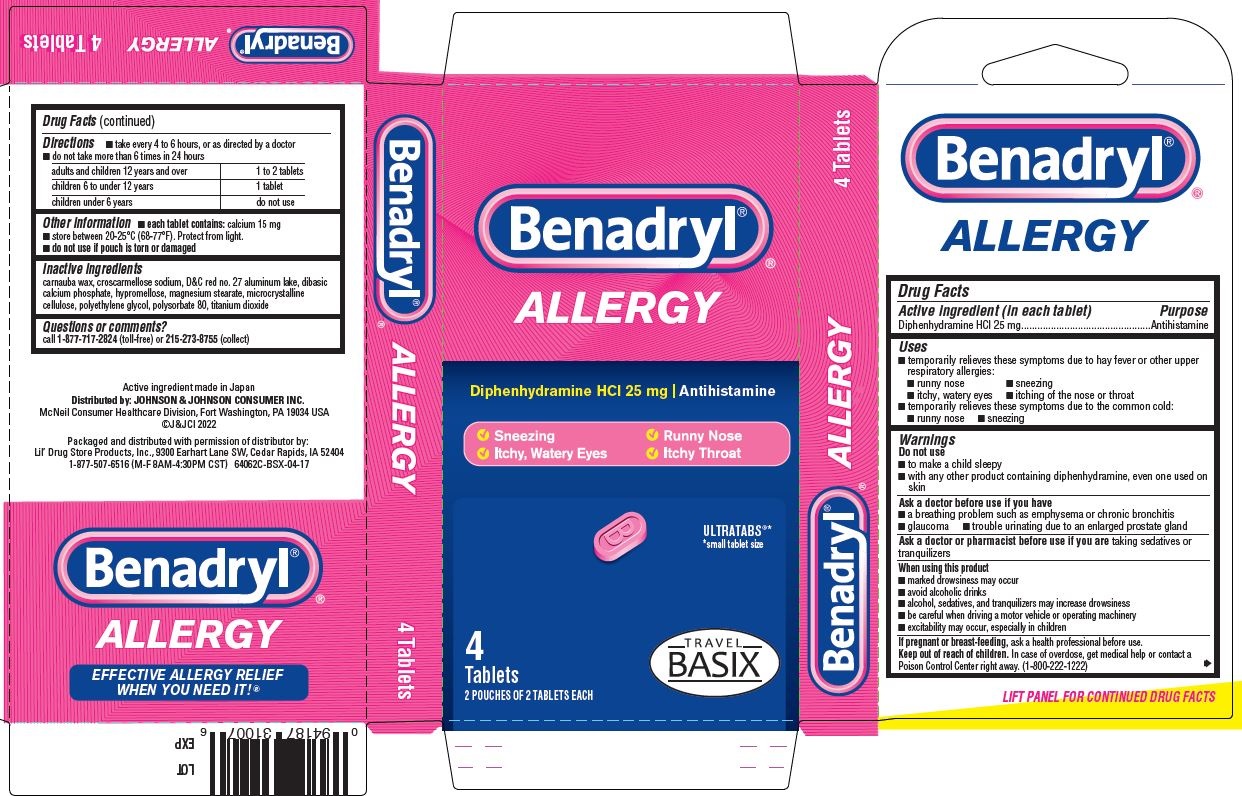
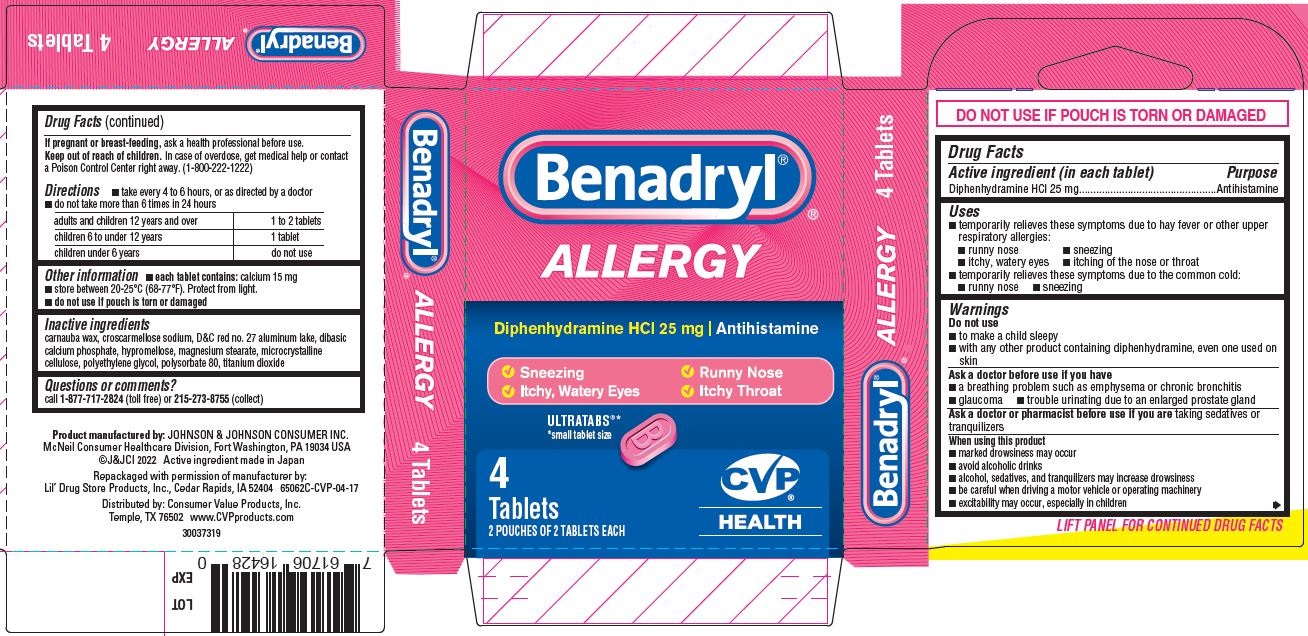
PRINCIPAL DISPLAY PANEL - 25 mg Tablet Pouch Carton
Benadryl ®
ALLERGY
Diphenhydramine HCl 25 mg | Antihistamine
- Sneezing
- Itchy, Watery Eyes
- Runny Nose
- Itchy Throat
ULTRATABS
®*
*small tablet size
6
Tablets
3 POUCHES OF 2 TABLETS EACH
Lil'
Drug Store
®