Rx only
To reduce the development of drug-resistant bacteria and maintain the effectiveness of Erythromycin Delayed-release Capsules, USP and other antibacterial drugs, Erythromycin Delayed-release Capsules, USP should be used only to treat or prevent infections that are proven or strongly suspected to be caused by bacteria.
DESCRIPTION
Erythromycin Delayed-release Capsules, USP contain enteric-coated pellets of erythromycin base for oral administration.
Each Erythromycin Delayed-release Capsule, USP contains 250 mg of erythromycin base.
Inactive Ingredients
Cellulosic polymers, citrate ester, D&C Red No. 30, D&C Yellow No. 10, magnesium stearate and povidone. The capsule shell contains FD&C Blue No. 1, FD&C Red No. 3, gelatin, and titanium dioxide.
Erythromycin is produced by a strain of Saccharopolyspora erythraea (formerly Streptomyces erythraeus) and belongs to the macrolide group of antibiotics. It is basic and readily forms salts with acids but it is the base which is microbiologically active. Erythromycin base is (3R*, 4S*, 5S*, 6R*, 7R*, 9R*, 11R*, 12R*, 13S*, 14R*)-4-[(2,6- Dideoxy-3-C-methyl-3-O-methyl-α-L-ribo-hexopyranosyl) oxy]-14-ethyl-7,12,13-trihydroxy-3,5,7,9,11,13-hexamethyl-6-[[3,4,6-trideoxy-3-(dimethylamino)-β-D-xylo-hexopyranosyl]oxy]oxacyclotetradecane-2,10-dione.
ERYTHROMYCIN
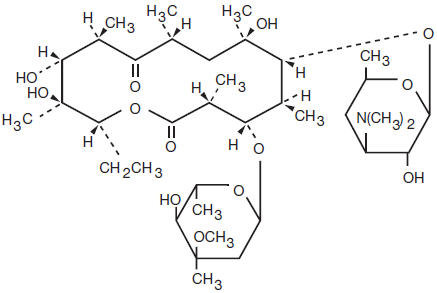
C37H67NO13 MW 734
CLINICAL PHARMACOLOGY
Orally administered erythromycin base and its salts are readily absorbed in the microbiologically active form. Interindividual variations in the absorption of erythromycin are, however, observed, and some patients do not achieve acceptable serum levels. Erythromycin is largely bound to plasma proteins, and the freely dissociating bound fraction after administration of erythromycin base represents 90 percent of the total erythromycin absorbed. After absorption, erythromycin diffuses readily into most body fluids. In the absence of meningeal inflammation, low concentrations are normally achieved in the spinal fluid, but the passage of the drug across the blood-brain barrier increases in meningitis. The drug is excreted in human milk. The drug crosses the placental barrier, but fetal plasma levels are low. Erythromycin is not removed by peritoneal dialysis or hemodialysis.
In the presence of normal hepatic function erythromycin is concentrated in the liver and is excreted in the bile; the effect of hepatic dysfunction on biliary excretion of erythromycin is not known. After oral administration, less than 5 percent of the administered dose can be recovered in the active form in the urine.
The enteric coating of pellets in Erythromycin Delayed release Capsules, USP protects the erythromycin base from inactivation by gastric acidity. Because of their small size and enteric coating, the pellets readily pass intact from the stomach to the small intestine and dissolve efficiently to allow absorption of erythromycin in a uniform manner. After administration of a single dose of a 250 mg Erythromycin Delayed-release Capsule, USP, peak serum levels in the range of 1.13 to 1.68 mcg/mL are attained in approximately 3 hours and decline to 0.30 to 0.42 mcg/mL in 6 hours. Optimal conditions for stability in the presence of gastric secretion and for complete absorption are attained when erythromycin is taken on an empty stomach.
Microbiology
Mechanism of Action
Erythromycin acts by inhibition of protein synthesis by binding 50 S ribosomal subunits of susceptible organisms. It does not affect nucleic acid synthesis.
Resistance
The major route of resistance is modification of the 23S rRNA in the 50S ribosomal subunit to insensitivity, while efflux can also be significant.
Interaction with other Antimicrobials
Antagonism exists in vitro between erythromycin and clindamycin, lincomycin, and chloramphenicol. Erythromycin has been shown to be active against most isolates of the following bacteria both in vitro and in clinical infections as described in the INDICATIONS AND USAGE section.
Gram-positive bacteria:
- Corynebacterium diphtheriae
- Corynebacterium minutissimum
- Listeria monocytogenes
- Staphylococcus aureus
- Streptococcus pneumoniae
- Streptococcus pyogenes
Gram-negative bacteria:
- Bordetella pertussis
- Haemophilus influenzae
- Legionella pneumophila
- Neisseria gonorrhoeae
Other Microorganisms:
- Chlamydia trachomatis
- Entamoeba histolytica
- Mycoplasma pneumoniae
- Treponema pallidum
- Ureaplasma urealyticum
INDICATIONS AND USAGE
Erythromycin is indicated in the treatment of infections caused by susceptible strains of the designated organisms in the diseases listed below:
Upper respiratory tract infections of mild to moderate degree caused by Streptococcus pyogenes, Streptococcus pneumoniae, or Haemophilus influenzae (when used concomitantly with adequate doses of sulfonamides, since many strains of H. influenzae are not susceptible to the erythromycin concentrations ordinarily achieved). (see appropriate sulfonamide labeling for prescribing information).
Lower respiratory tract infections of mild to moderate severity caused by Streptococcus pneumoniae or Streptococcus pyogenes.
Listeriosis caused by Listeria monocytogenes.
Pertussis (whooping cough) caused by Bordetella pertussis. Erythromycin is effective in eliminating the organism from the nasopharynx of infected individuals rendering them noninfectious. Some clinical studies suggest that erythromycin may be helpful in the prophylaxis of pertussis in exposed susceptible individuals.
Respiratory tract infections due to Mycoplasma pneumoniae.
Skin and skin structure infections of mild to moderate severity caused by Streptococcus pyogenes or Staphylococcus aureus (resistant staphylococci may emerge during treatment).
Diphtheria: Infections due to Corynebacterium diphtheriae, as an adjunct to antitoxin, to prevent establishment of carriers and to eradicate the organism in carriers.
Erythrasma: In the treatment of infections due to Corynebacterium minutissimum.
Syphilis caused by Treponema pallidum: Erythromycin is an alternate choice of treatment for primary syphilis in penicillin-allergic patients. In primary syphilis, spinal fluid examinations should be done before treatment and as part of follow-up after therapy.
Intestinal amebiasis caused by Entamoeba histolytica (oral erythromycins only). Extraenteric amebiasis requires treatment with other agents.
Acute pelvic inflammatory disease caused by Neisseria gonorrhoeae: Erythromycin lactobionate for injection, USP followed by erythromycin base orally, as an alternative drug in treatment of acute pelvic inflammatory disease caused by N. gonorrhoeae in female patients with a history of sensitivity to penicillin. Patients should have a serologic test for syphilis before receiving erythromycin as treatment of gonorrhea and a follow-up serologic test for syphilis after 3 months.
Erythromycins are indicated for the treatment of the following infections caused by Chlamydia trachomatis: conjunctivitis of the newborn, pneumonia of infancy, and urogenital infections during pregnancy. When tetracyclines are contraindicated or not tolerated, erythromycin is indicated for the treatment of uncomplicated urethral, endocervical, or rectal infections in adults due to Chlamydia trachomatis.
When tetracyclines are contraindicated or not tolerated, erythromycin is indicated for the treatment of nongonococcal urethritis caused by Ureaplasma urealyticum.
Legionnaires' Disease caused by Legionella pneumophila. Although no controlled clinical efficacy studies have been conducted, in vitro and limited preliminary clinical data suggest that erythromycin may be effective in treating Legionnaires' Disease.
Prophylaxis
Prevention of Initial Attacks of Rheumatic Fever
Penicillin is considered by the American Heart Association to be the drug of choice in the prevention of initial attacks of rheumatic fever (treatment of Streptococcus pyogenes infections of the upper respiratory tract, e.g., tonsillitis or pharyngitis). Erythromycin is indicated for the treatment of penicillin-allergic patients.1 The therapeutic dose should be administered for ten days.
Prevention of Recurrent Attacks of Rheumatic Fever
Penicillin or sulfonamides are considered by the American Heart Association to be the drugs of choice in the prevention of recurrent attacks of rheumatic fever. In patients who are allergic to penicillin and sulfonamides, oral erythromycin is recommended by the American Heart Association in the long-term prophylaxis of streptococcal pharyngitis (for the prevention of recurrent attacks of rheumatic fever).1
To reduce the development of drug-resistant bacteria and maintain the effectiveness of Erythromycin Delayed-release Capsules, USP and other antibacterial drugs, Erythromycin Delayed-release Capsules, USP should be used only to treat or prevent infections that are proven or strongly suspected to be caused by susceptible bacteria. When culture and susceptibility information are available, they should be considered in selecting or modifying antibacterial therapy. In the absence of such data, local epidemiology and susceptibility patterns may contribute to the empiric selection of therapy.
CONTRAINDICATIONS
Erythromycin is contraindicated in patients with known hypersensitivity to this antibiotic.
Erythromycin is contraindicated in patients taking terfenadine, astemizole, cisapride, pimozide, ergotamine, or dihydroergotamine (see PRECAUTIONS - Drug Interactions).
Do not use erythromycin concomitantly with HMG CoA reductase inhibitors (statins) that are extensively metabolized by CYP 3A4 (lovastatin or simvastatin), due to the increased risk of myopathy, including rhabdomyolysis (see PRECAUTIONS - Drug Interactions).
WARNINGS
Hepatotoxicity
There have been reports of hepatic dysfunction, including increased liver enzymes, and hepatocellular and/or cholestatic hepatitis, with or without jaundice, occurring in patients receiving oral erythromycin products.
QT Prolongation
Erythromycin has been associated with prolongation of the QT interval and infrequent cases of arrhythmia. Cases of torsades de pointes have been spontaneously reported during postmarketing surveillance in patients receiving erythromycin. Fatalities have been reported. Erythromycin should be avoided in patients with known prolongation of the QT interval, patients with ongoing proarrhythmic conditions such as uncorrected hypokalemia or hypomagnesemia, clinically significant bradycardia, and in patients receiving Class IA (quinidine, procainamide) or Class III (dofetilide, amiodarone, sotalol) antiarrhythmic agents. Elderly patients may be more susceptible to drug-associated effects on the QT interval.
Syphilis in pregnancy
There have been reports suggesting that erythromycin does not reach the fetus in adequate concentration to prevent congenital syphilis. Infants born to women treated during pregnancy with oral erythromycin for early syphilis should be treated with an appropriate penicillin regimen.
Clostridium difficile-associated diarrhea
Clostridium difficile-associated diarrhea (CDAD) has been reported with use of nearly all antibacterial agents, including Erythromycin Delayed-release Capsules, USP, and may range in severity from mild diarrhea to fatal colitis. Treatment with antibacterial agents alters the normal flora of the colon leading to overgrowth of C. difficile.
C. difficile produces toxins A and B which contribute to the development of CDAD. Hypertoxin producing strains of C. difficile cause increased morbidity and mortality, as these infections can be refractory to antimicrobial therapy and may require colectomy. CDAD must be considered in all patients who present with diarrhea following antibiotic use. Careful medical history is necessary since CDAD has been reported to occur over two months after the administration of antibacterial agents.
If CDAD is suspected or confirmed, ongoing antibiotic use not directed against C. difficile may need to be discontinued. Appropriate fluid and electrolyte management, protein supplementation, antibiotic treatment of C. difficile, and surgical evaluation should be instituted as clinically indicated.
Drug Interactions
Serious adverse reactions have been reported in patients taking erythromycin concomitantly with CYP3A4 substrates. These include colchicine toxicity with colchicine; rhabdomyolysis with simvastatin, lovastatin, and atorvastatin; and hypotension with calcium channel blockers metabolized by CYP3A4 (for example, verapamil, amlodipine, diltiazem) (see PRECAUTIONS, Drug Interactions).
There have been post-marketing reports of colchicine toxicity with concomitant use of erythromycin and colchicine. This interaction is potentially life-threatening, and may occur while using both drugs at their recommended doses (see PRECAUTIONS, Drug Interactions).
Rhabdomyolysis with or without renal impairment has been reported in seriously ill patients receiving erythromycin concomitantly with lovastatin. Therefore, patients receiving concomitant lovastatin and erythromycin should be carefully monitored for creatine kinase (CK) and serum transaminase levels. (See package insert for lovastatin.)
PRECAUTIONS
General
Prescribing Erythromycin Delayed-release Capsules, USP in the absence of a proven or strongly suspected bacterial infection or a prophylactic indication is unlikely to provide benefit to the patient and increases the risk of the development of drug-resistant bacteria.
Since erythromycin is principally excreted by the liver, caution should be exercised when erythromycin is administered to patients with impaired hepatic function (see CLINICAL PHARMACOLOGY and WARNINGS).
Exacerbation of symptoms of myasthenia gravis and new onset of symptoms of myasthenic syndrome has been reported in patients receiving erythromycin therapy.
There have been reports of infantile hypertrophic pyloric stenosis (IHPS) occurring in infants following erythromycin therapy. In one cohort of 157 newborns who were given erythromycin for pertussis prophylaxis, seven neonates (5 percent) developed symptoms of non bilious vomiting or irritability with feeding and were subsequently diagnosed as having IHPS requiring surgical pyloromyotomy. A possible dose-response effect was described with an absolute risk of IHPS of 5.1 percent for infants who took erythromycin for 8 to 14 days and 10 percent for infants who took erythromycin for 15 to 21 days.2
Since erythromycin may be used in the treatment of conditions in infants which are associated with significant mortality or morbidity (such as pertussis or neonatal Chlamydia trachomatis infections), the benefit of erythromycin therapy needs to be weighed against the potential risk of developing IHPS. Parents should be informed to contact their physician if vomiting or irritability with feeding occurs.
Prolonged or repeated use of erythromycin may result in an overgrowth of nonsusceptible bacteria or fungi. If superinfection occurs, erythromycin should be discontinued and appropriate therapy instituted.
When indicated, incision and drainage or other surgical procedures should be performed in conjunction with antibiotic therapy.
Information for Patients
Patients should be counseled that antibacterial drugs including Erythromycin Delayed-release Capsules, USP should only be used to treat bacterial infections. They do not treat viral infections (for example, the common cold). When Erythromycin Delayed-release Capsules, USP are prescribed to treat a bacterial infection, patients should be told that although it is common to feel better early in the course of therapy, the medication should be taken exactly as directed. Skipping doses or not completing the full course of therapy may (1) decrease the effectiveness of the immediate treatment and (2) increase the likelihood that bacteria will develop resistance and will not be treatable by Erythromycin Delayed-release Capsules, USP or other antibacterial drugs in the future.
Diarrhea is a common problem caused by antibiotics which usually ends when the antibiotic is discontinued. Sometimes after starting treatment with antibiotics, patients can develop watery and bloody stools (with or without stomach cramps and fever) even as late as two or more months after having taken the last dose of the antibiotic. If this occurs, patients should contact their physician as soon as possible.
Drug Interactions
Theophylline
Erythromycin use in patients who are receiving high doses of theophylline may be associated with an increase in serum theophylline levels and potential theophylline toxicity. In case of theophylline toxicity and/or elevated serum theophylline levels, the dose of theophylline should be reduced while the patient is receiving concomitant erythromycin therapy.
There have been published reports suggesting that when oral erythromycin is given concurrently with theophylline there is a significant decrease in erythromycin serum concentrations of approximately 35 percent. The mechanism by which this interaction occurs is unknown. The decrease in erythromycin concentrations due to co-administration of theophylline could result in subtherapeutic concentrations of erythromycin.
Hypotension, bradyarrhythmias, and lactic acidosis have been observed in patients receiving concurrent verapamil, belonging to the calcium channel blockers drug class.
Concomitant administration of erythromycin and digoxin has been reported to result in elevated digoxin serum levels.
There have been reports of increased anticoagulant effects when erythromycin and oral anticoagulants were used concomitantly. Increased anticoagulation effects due to interactions of erythromycin with oral anticoagulants may be more pronounced in the elderly.
Erythromycin is a substrate and inhibitor of the 3A isoform subfamily of the cytochrome P450 enzyme system (CYP3A). Co-administration of erythromycin and a drug primarily metabolized by CYP3A may be associated with elevations in drug concentrations that could increase or prolong both the therapeutic and adverse effects of the concomitant drug. Dosage adjustments may be considered, and when possible, serum concentrations of drugs primarily metabolized by CYP3A should be monitored closely in patients concurrently receiving erythromycin.
The following are examples of some clinically significant CYP3A based drug interactions. Interactions with other drugs metabolized by the CYP3A isoform are also possible. The following CYP3A based drug interactions have been observed with erythromycin products in post-marketing experience:
Ergotamine/dihydroergotamine
Post-marketing reports indicate that co-administration of erythromycin with ergotamine or dihydroergotamine has been associated with acute ergot toxicity characterized by vasospasm and ischemia of the extremities and other tissues including central nervous system. Concomitant administration of erythromycin with ergotamine or dihydroergotamine is contraindicated (see CONTRAINDICATIONS).
Triazolobenzodiazepines (such as triazolam and alprazolam) and related benzodiazepines
Erythromycin has been reported to decrease the clearance of triazolam and midazolam, and thus, may increase the pharmacologic effect of these benzodiazepines.
HMG-CoA Reductase Inhibitors
Erythromycin has been reported to increase concentrations of HMG-CoA reductase inhibitors (for example, lovastatin and simvastatin). Rare reports of rhabdomyolysis have been reported in patients taking these drugs concomitantly (see CONTRAINDICATIONS).
Sildenafil (Viagra)
Erythromycin has been reported to increase the systemic exposure (AUC) of sildenafil. Reduction of sildenafil dosage should be considered. (See Viagra package insert.)
There have been spontaneous or published reports of CYP3A based interactions of erythromycin with carbamazepine, cyclosporine, tacrolimus, alfentanil, disopyramide, bromocriptine, rifabutin, quinidine, methylprednisolone, cilostazol, and vinblastine.
Concomitant administration of erythromycin with cisapride, pimozide, astemizole, or terfenadine is contraindicated. (see CONTRAINDICATIONS).
In addition, there have been reports of interactions of erythromycin with drugs not thought to be metabolized by CYP3A, including hexobarbital, phenytoin, and valproate.
Erythromycin has been reported to significantly alter the metabolism of the nonsedating antihistamines terfenadine and astemizole when taken concomitantly. Rare cases of serious cardiovascular adverse events, including electrocardiographic QT/QTc interval prolongation, cardiac arrest, torsades de pointes, and other ventricular arrhythmias, have been observed (see CONTRAINDICATIONS). In addition, deaths have been reported rarely with concomitant administration of terfenadine and erythromycin.
There have been post-marketing reports of drug interactions when erythromycin was co-administered with cisapride, resulting in QT prolongation, cardiac arrhythmias, ventricular tachycardia, ventricular fibrillation, and torsades de pointes, most likely due to the inhibition of hepatic metabolism of cisapride by erythromycin. Fatalities have been reported (see CONTRAINDICATIONS).
Colchicine
Colchicine is a substrate for both CYP3A4 and the efflux transporter P-glycoprotein (P-gp). Erythromycin is considered a moderate inhibitor of CYP3A4. A significant increase in colchicine plasma concentration is anticipated when co-administered with moderate CYP3A4 inhibitors such as erythromycin. If co-administration of colchicine and erythromycin is necessary, the starting dose of colchicine may need to be reduced, and the maximum colchicine dose should be lowered. Patients should be monitored for clinical symptoms of colchicine toxicity (see WARNINGS).
Drug/Laboratory test interactions
Erythromycin interferes with the fluorometric determination of urinary catecholamines.
Carcinogenesis, Mutagenesis and Impairment of Fertility
Long-term (2-year) oral studies conducted in rats with erythromycin base did not provide evidence of tumorigenicity. Mutagenicity studies have not been conducted. There was no apparent effect on male or female fertility in rats fed erythromycin (base) at levels up to 0.25 percent of diet.
Pregnancy
Teratogenic Effects
There was no evidence of teratogenicity or any other adverse effect on reproduction in female rats fed erythromycin base (up to 0.25 percent of diet) prior to and during mating, during gestation, and through weaning of two successive litters. There are, however, no adequate and well-controlled studies in pregnant women. Because animal reproduction studies are not always predictive of human response, this drug should be used during pregnancy only if clearly needed.
Nursing Mothers
Erythromycin is excreted in human milk. Caution should be exercised when erythromycin is administered to a nursing woman.
Geriatric Use
Clinical studies with Erythromycin Delayed-release Capsules, USP did not include sufficient numbers of subjects aged 65 and over to determine whether they respond differently from younger subjects. Other reported clinical experience has not identified differences in responses between the elderly and younger patients. In general, dose selection for an elderly patient should be cautious, usually starting at the low end of the dosing range, reflecting the greater frequency of the decreased hepatic, renal, or cardiac function, and of concomitant disease or other drug therapy.
Elderly patients may be more susceptible to development of torsades de pointes arrhythmias than younger patients. (see WARNINGS).
Elderly patients may experience increased effects of oral anticoagulant therapy while undergoing treatment with erythromycin (see PRECAUTIONS, Drug Interactions).
Erythromycin Delayed-release Capsules, USP 250 mg do not contain sodium or potassium.
ADVERSE REACTIONS
The most frequent side effects of oral erythromycin preparations are gastrointestinal and are dose-related. They include nausea, vomiting, abdominal pain, diarrhea and anorexia. Symptoms of hepatitis, hepatic dysfunction and/or abnormal liver function test results may occur (see WARNINGS).
Onset of pseudomembranous colitis symptoms may occur during or after antibacterial treatment (see WARNINGS).
Erythromycin has been associated with QT prolongation and ventricular arrhythmias, including ventricular tachycardia and torsade de pointes (see WARNINGS).
Allergic reactions ranging from urticaria to anaphylaxis have occurred. Skin reactions ranging from mild eruptions to erythema multiforme, Stevens-Johnson syndrome, and toxic epidermal necrolysis have been reported rarely.
There have been reports of interstitial nephritis coincident with erythromycin use.
There have been reports of pancreatitis and convulsions.
There have been isolated reports of reversible hearing loss occurring chiefly in patients with renal insufficiency and in patients receiving high doses of erythromycin.
OVERDOSAGE
In case of overdosage, erythromycin should be discontinued. Overdosage should be handled with the prompt elimination of unabsorbed drug and all other appropriate measures.
Erythromycin is not removed by peritoneal dialysis or hemodialysis.
DOSAGE AND ADMINISTRATION
Erythromycin is well absorbed and may be given without regard to meals. Optimum blood levels are obtained in a fasting state (administration at least one half hour and preferably two hours before or after a meal); however, blood levels obtained upon administration of enteric-coated erythromycin products in the presence of food are still above minimal inhibitory concentrations (MICs) of most organisms for which erythromycin is indicated.
ADULTS
The usual dose is 250 mg every 6 hours taken one hour before meals. If twice-a-day dosage is desired, the recommended dose is 500 mg every 12 hours. Dosage may be increased up to 4 grams per day, according to the severity of the infection. Twice-a-day dosing is not recommended when doses larger than 1 gram daily are administered.
CHILDREN
Age, weight, and severity of the infection are important factors in determining the proper dosage. The usual dosage is 30 to 50 mg/kg/day in divided doses. For the treatment of more severe infections, this dose may be doubled.
Streptococcal infections
A therapeutic dosage of oral erythromycin should be administered for at least 10 days. For continuous prophylaxis against recurrences of streptococcal infections in persons with a history of rheumatic heart disease, the dose is 250 mg twice a day.
Intestinal amebiasis
250 mg four times daily for 10 to 14 days for adults; 30 to 50 mg/kg/day in divided doses for 10 to 14 days for children.
Legionnaires' disease
Although optimal doses have not been established, doses utilized in reported clinical data were those recommended above (1 to 4 grams daily in divided doses).
Urogenital infections during pregnancy due to Chlamydia trachomatis
Although the optimal dose and duration of therapy have not been established, the suggested treatment is erythromycin 500 mg, by mouth, 4 times a day on an empty stomach for at least 7 days. For women who cannot tolerate this regimen, a decreased dose of 250 mg, by mouth, 4 times a day should be used for at least 14 days.
For adults with uncomplicated urethral, endocervical, or rectal infections caused by Chlamydia trachomatis in whom tetracyclines are contraindicated or not tolerated: 500 mg, by mouth, 4 times a day for at least 7 days.
Pertussis
Although optimum dosage and duration of therapy have not been established, doses of erythromycin utilized in reported clinical studies were 40 to 50 mg/kg/day, given in divided doses for 5 to 14 days.
HOW SUPPLIED
Erythromycin Delayed-release Capsules, USP, are clear and opaque maroon capsules bearing the Product Code ER with pink and yellow particles containing 250 mg of erythromycin supplied in:
Bottles of 100 (NDC 24338-120-13)
REFERENCES
- Committee on Rheumatic Fever, Endocarditis, and Kawasaki Disease of the Council on Cardiovascular Disease in the Young, the American Heart Association: Prevention of Rheumatic Fever. Circulation. 78(4):1082-1086, October 1988.
- Honein, M.A., et al.: Infantile hypertrophic pyloric stenosis after pertussis prophylaxis with erythromycin: a case review and cohort study. The Lancet 1999;354 (9196): 2101-5.
