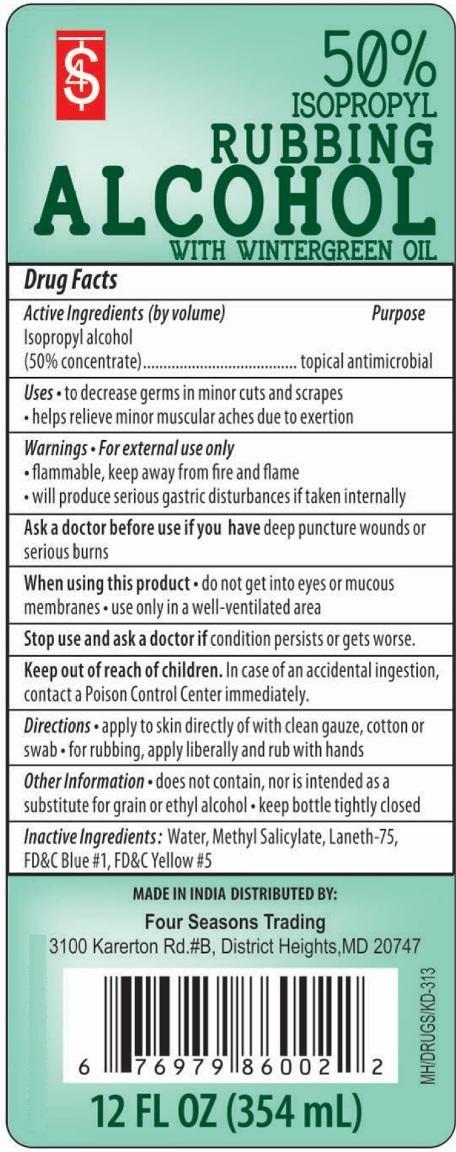
ISOPROPYL RUBBING ALCOHOL 50% WITH WINTERGREEN- isopropyl alcohol liquid
FOUR SEASONS TRADING INC
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Active Ingredients (by volume)
Isopropyl alcohol (50% concentrate)
Purpose
topical antimicrobial
Uses
- to decrease germs in minor cuts and scrapes
- helps relieve minor muscular aches due to exertion
Warnings
For external use only
- flammable, keep away from fire and flame
- will produce serious gastric disturbances if taken internally
Ask a doctor before use if you have deep puncture wounds or serious burns.
When using this product
- do not get into eyes or mucous membranes
- use only in a well-ventilated area
Stop use and ask a doctor if condition persists or gets worse.
Keep out of reach of children
In case of an accidental ingestion, contact a Poison Control Center immediately.
Directions
- apply to skin directly of with clean gauze, cotton or swab
- for rubbing apply liberally and rub with hands
Other information
- does not contain, nor is intended as a substitute for grain or ethyl alcohol
- keep bottle tightly closed
Inactive Ingredients
Water, Methyl Salicylate, Laneth-75, FD&C Blue #1, FD&C Yellow #5.
PRINCIPAL DISPLAY PANEL
ISOPROPYL RUBBING ALCOHOL 50% WITH WINTERGREEN
TOPICAL ANTIMICROBIAL
12 FL.OZ (354 mL)