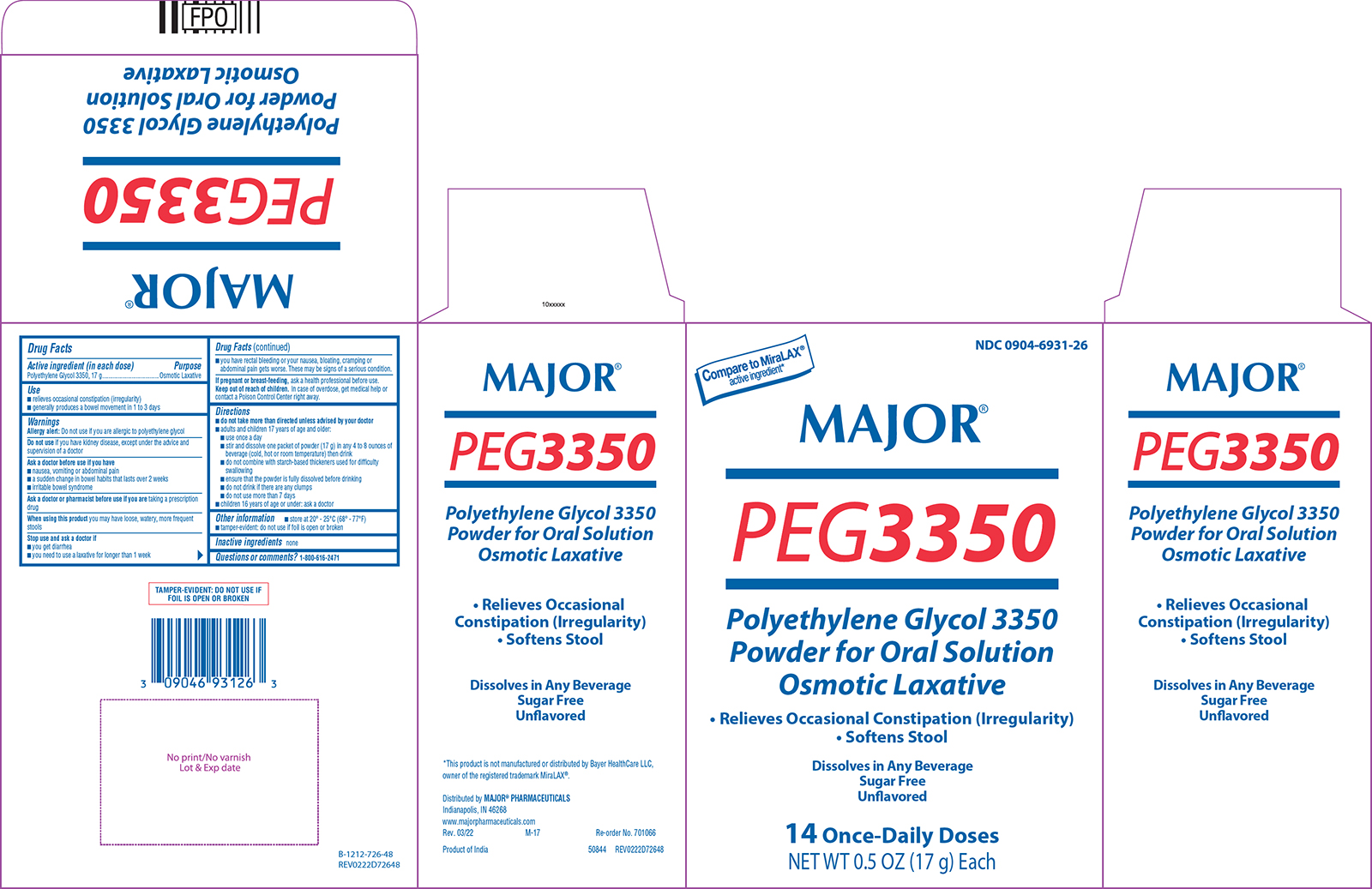
Use
- relieves occasional constipation (irregularity)
- generally produces a bowel movement in 1 to 3 days
Warnings
Allergy alert: Do not use if you are allergic to polyethylene glycol
Ask a doctor before use if you have
- nausea, vomiting or abdominal pain
- a sudden change in bowel habits that lasts over 2 weeks
- irritable bowel syndrome
Directions
- do not take more than directed unless advised by your doctor
- adults and children 17 years of age and older:
- use once a day
- stir and dissolve one packet of powder (17 g) in any 4 to 8 ounces of beverage (cold, hot or room temperature) then drink
- do not combine with starch-based thickeners used for difficulty swallowing
- ensure that the powder is fully dissolved before drinking
- do not drink if there are any clumps
- do not use more than 7 days
- children 16 years of age or under: ask a doctor
Other information
- store at 20° - 25°C (68° - 77°F)
- tamper-evident: do not use if foil is open or broken
Principal display panel
NDC 0904-6931-26
Compare to MiraLAX®
active ingredient*
MAJOR®
PEG3350
Polyethylene Glycol 3350
Powder for Oral Solution
Osmotic Laxative
• Relieves Occasional Constipation (Irregularity)
• Softens Stool
Dissolves in Any Beverage
Sugar Free
Unflavored
14 Once-Daily Doses
NET WT 0.5 OZ (17 g) Each
TAMPER-EVIDENT: DO NOT USE IF
FOIL IS OPEN OR BROKEN
*This product is not manufactured or distributed by Bayer HealthCare LLC,
owner of the registered trademark MiraLAX®.
Distributed by MAJOR® PHARMACEUTICALS
Indianapolis, IN 46268
www.majorpharmaceuticals.com
Rev. 03/22 M-17 Re-order No. 701066
Product of India 50844 REV0222D72648
Major 44-726