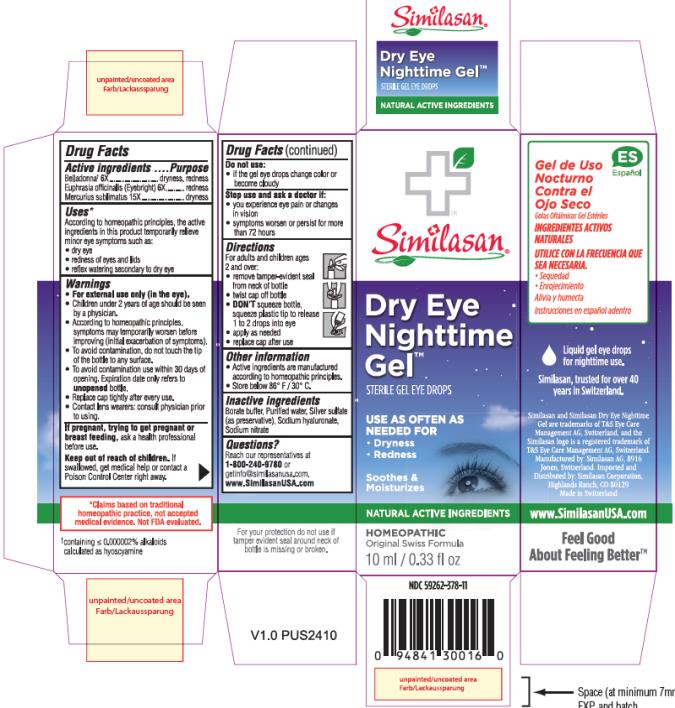
Uses:
According to homeopathic principles, the active ingredients in this medication temporarily relieve minor symptoms such as:
- dry eye
- redness of eyes and lids
- reflex watering secondary to dry eye
Warnings:
-
For external use only (in the eye).
- Children under 2 years of age should be seen by a physician.
- According to homeopathic principles, symptoms may temporarily worsen before improving (initial exacerbation of symptoms).
- To avoid contamination, do not touch the tip of the container to any surface.
- To avoid contamination use within 30 days of opening. Expiration date only refers to unopened bottle.
- Replace cap tightly after every use.
- Contact lens wearers: consult physician prior to using.
Directions:
For adults and children age 2 and over:
- remove tamper-evident seal from neck of bottle
- twist cap off bottle
-
DON’T squeeze bottle, squeeze plastic tip to release 1 to 2 drops into eye
- apply as needed
- replace cap after use
Other Information:
- Active ingredients are manufactured according to homeopathic principles.
- Store below 86° F / 30° C
Inactive Ingredients
Borate buffer, Purified water, Silver sulphate (as preservative), Sodium hyaluronate, Sodium nitrate
Questions?
Reach our representatives at 1-800-240-9780 or getinfo@similasanusa.com.
www.SimilasanUSA.com
*containing ≤0.000002% alkaloids calculated as hyoscyamine