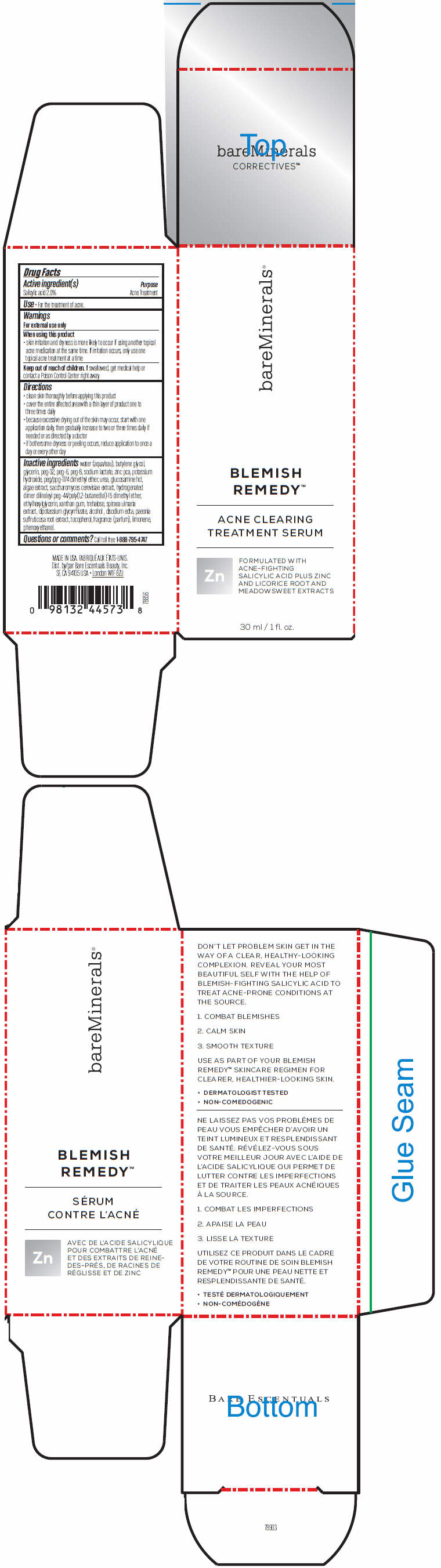
BAREMINERALS BLEMISH REMEDY ACNE CLEARING TREATMENT SERUM- salicylic acid emulsion
Bare Escentuals Beauty, Inc.
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Active ingredient(s)
Salicylic acid 2.0%
Use
- For the treatment of acne.
Warnings
For external use only
When using this product:
- skin irritation and dryness is more likely to occur if using another topical acne medication at the same time. If irritation occurs, only use one topical acne treatment at a time
Keep out of reach of children. If swallowed, get medical help or contact a Poison Control Center right away.
Directions
- clean skin thoroughly before applying this product
- cover the entire affected area with a thin layer of product one to three times daily
- because excessive drying out of the skin may occur, start with one application daily, then gradually increase to two or three times daily if needed or as directed by a doctor
- if bothersome dryness or peeling occurs, reduce application to once a day or every other day
Inactive ingredients
water (aqua/eau), butylene glycol, glycerin, peg-32, peg-6, peg-8, sodium lactate, zinc pca, potassium hydroxide, peg/ppg-17/4 dimethyl ether, urea, glucosamine hcl, algae extract, saccharomyces cerevisiae extract, hydrogenated dimer dilinoleyl peg-44/poly(1,2-butanediol)-15 dimethyl ether, ethylhexylglycerin, xanthan gum, trehalose, spiraea ulmaria extract, dipotassium glycyrrhizate, alcohol , disodium edta, paeonia suffruticosa root extract, tocopherol, fragrance (parfum), limonene, phenoxyethanol.
Questions or comments?
Call toll free 1-888-795-4747
Dist. by Bare Escentuals Beauty, Inc.
SF, CA 94105 USA
PRINCIPAL DISPLAY PANEL - 30 ml Bottle Carton
bareMinerals®
BLEMISH
REMEDY™
ACNE CLEARING
TREATMENT SERUM
Zn
FORMULATED WITH
ACNE-FIGHTING
SALICYLIC ACID PLUS ZINC
AND LICORICE ROOT AND
MEADOWSWEET EXTRACTS
30 ml / 1 fl. oz.
Bare Escentuals Beauty, Inc.