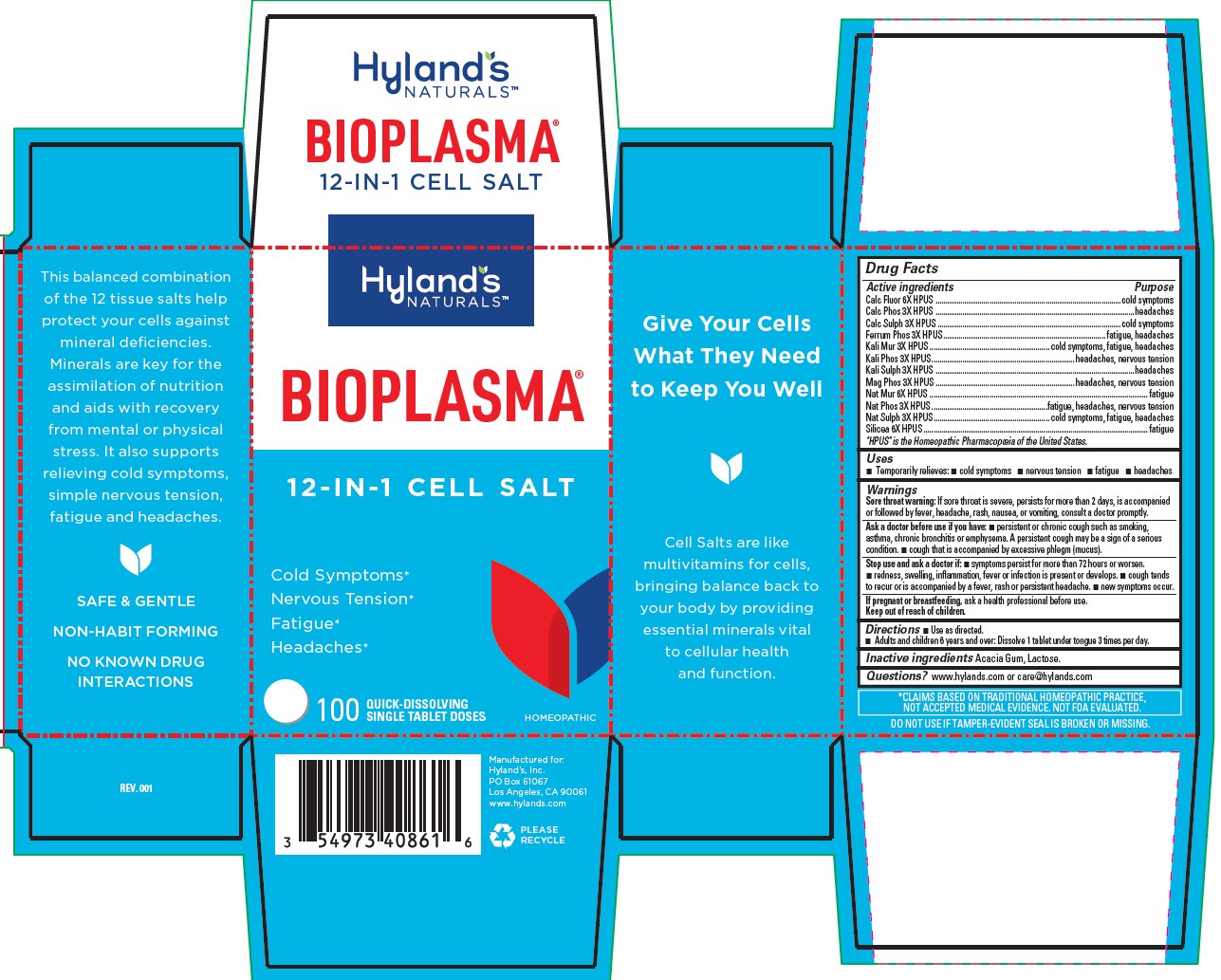
BIOPLASMA- silicon dioxide,potassium chloride,sodium sulfate,ferrosoferric phosphate,magnesium phosphate, dibasic trihydrate,sodium phosphate, dibasic, heptahydrate,tribasic calcium phosphate,potassium phosphate, dibasic,potassium sulfate,sodium chloride,calcium sulfate anhydrous,calcium fluoride tablet, soluble
Hyland's Inc.
Disclaimer: This homeopathic product has not been evaluated by the Food and Drug Administration for safety or efficacy. FDA is not aware of scientific evidence to support homeopathy as effective.
----------
Drug Facts
| Active ingredients | Purpose |
| Calc Fluor 6X HPUS | cold symptoms |
| Calc Phos 3X HPUS | headaches |
| Calc Sulph 3X HPUS | cold symptoms |
| Ferrum Phos 3X HPUS | fatigue, headaches |
| Kali Mur 3X HPUS | cold symptoms, fatigue, headaches |
| Kali Phos 3X HPUS | headaches, nervous tension |
| Kali Sulph 3X HPUS | headaches |
| Mag Phos 3X HPUS | headaches, nervous tension |
| Nat Mur 6X HPUS | fatigue |
| Nat Phos 3X HPUS | fatigue, headaches, nervous tension |
| Nat Sulph 3X HPUS | cold symptoms, fatigue, headaches |
| Silicea 6X HPUS | fatigue |
"HPUS" is the Homeopathic Pharmacopoeia of the United States.
Uses
■ Temporarily relieves: ■ cold symptoms ■ nervous tension ■ fatigue ■ headaches
Warnings
Sore throat warning
If sore throat is severe, persists for more than 2 days, is accompanied or followed by fever, headache, rash, nausea, or vomiting, consult a doctor promptly.
Ask a doctor before use if you have
■ persistent or chronic cough such as smoking, asthma, chronic bronchitis or emphysema. A persistent cough may be a sign of a serious condition. ■ cough that is accompanied by excessive phlegm (mucus).
Stop use and ask a doctor if
■ symptoms persist for more than 72 hours or worsen. ■ redness, swelling, inflammation, fever or infection is present or develops.
■ cough tends to recur or is accompanied by a fever, rash or persistent headache. ■ new symptoms occur.
If pregnant or breastfeeding
Ask a health professional before use.
Keep out of reach of children.
Directions
■ Use as directed.
■ Adults and children 6 years and over: Dissolve 1 tablet under tongue 3 times per day.
Inactive ingredients
Acacia Gum, Lactose.
Questions?
www.hylands.com or care@hylands.com
*CLAIMS BASED ON TRADITIONAL HOMEOPATHIC PRACTICE, NOT ACCEPTED MEDICAL EVIDENCE. NOT FDA EVALUATED.
DO NOT USE IF TAMPER-EVIDENT SEAL IS BROKEN OR MISSING.
Purpose
Cold Symptoms
Nervous Tension
Fatigue
Headaches
PRINCIPAL DISPLAY PANEL
Hyland's
NATURALS™
BIOPLASMA
®
12 - IN - 1 CELL SALT
Cold Symptoms*
Nervous Tension*
Fatigue*
Headaches*
100
QUICK-DISSOLVING
SINGLE TABLET DOSES
HOMEOPATHIC
Hyland's Inc.
