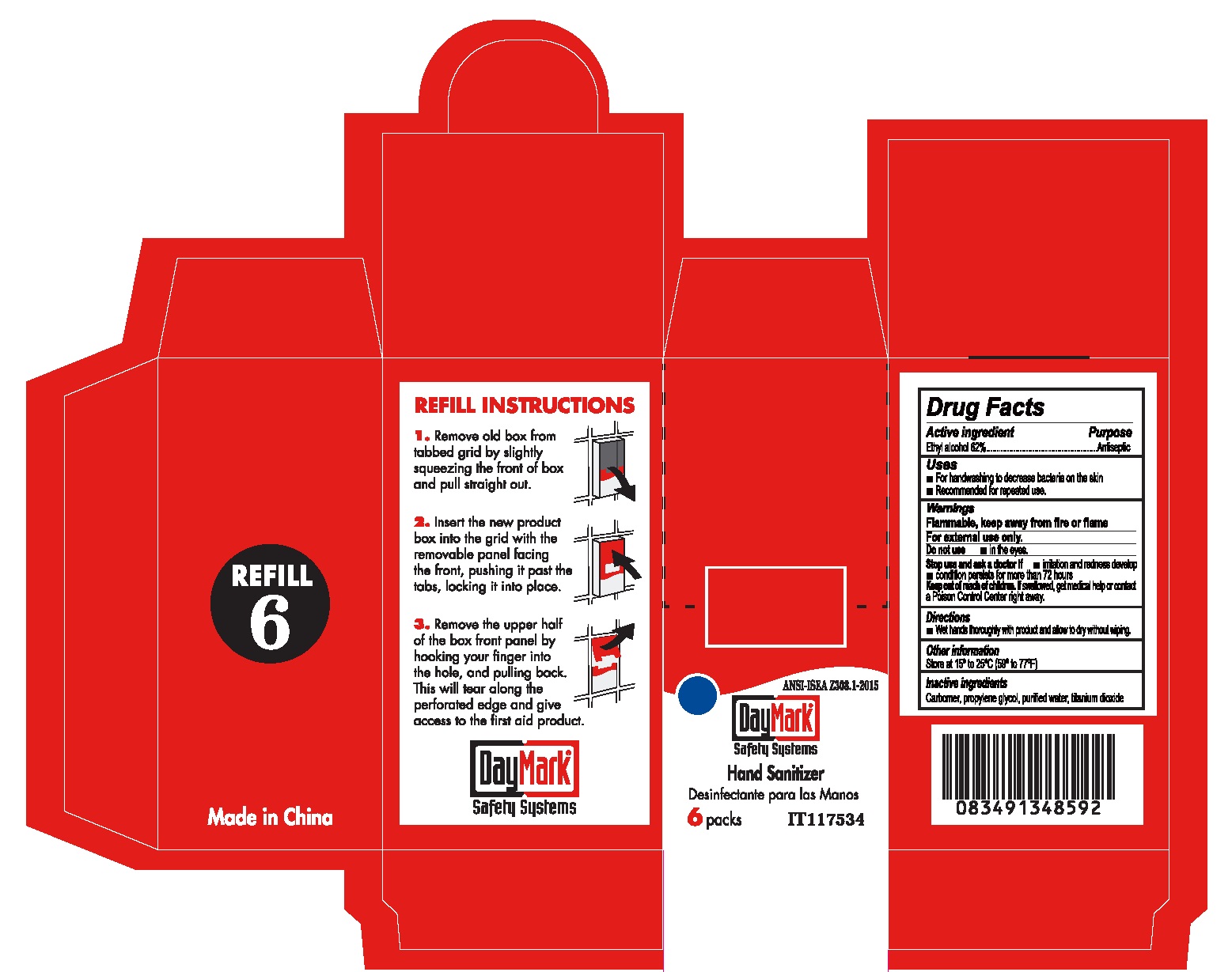
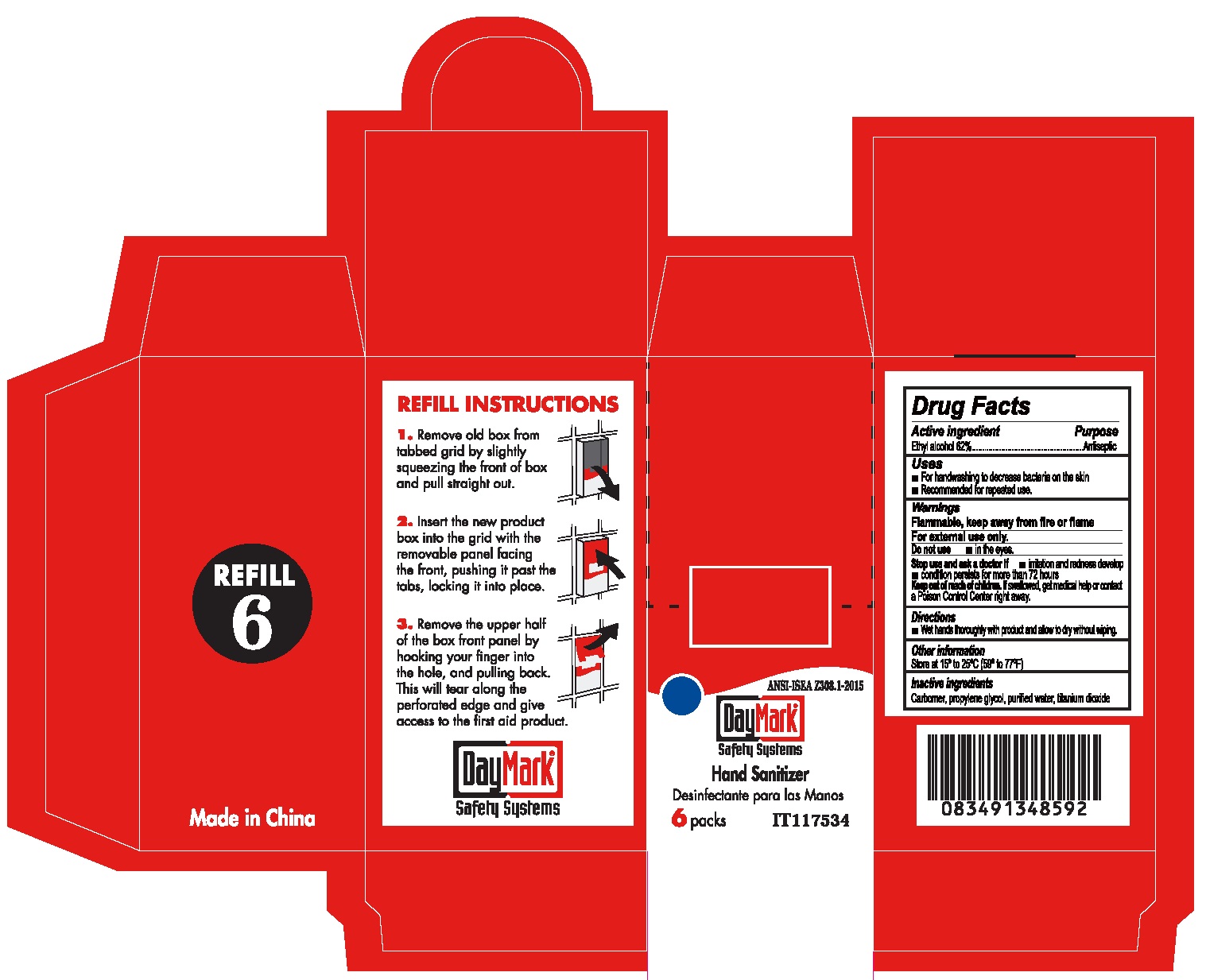
HAND SANITIZER- alcohol gel
CMC Group Inc.
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Active ingredient
Ethyl alcohol 62%
Uses
- For handwashing to decrease bacteria on the skin
- Recommended for repeated use.
Warnings
Flammable, keep away from fire or flame
For external use only.
Stop use and ask a doctor if
- irritation and redness develop
- condition persists for more than 72 hours
Keep out of reach children.
If swallowed, get medical help or contact a Poison Control Center right away.
Directions
- Wet hands thoroughly with product and allow to dry without wiping.
Other information
Store at 15° to 25°C (59° to 77°F)
Inactive ingredients
Carbomer, propylene glycol, purified water, titanium dioxide
Package Labeling
CMC Group Inc.