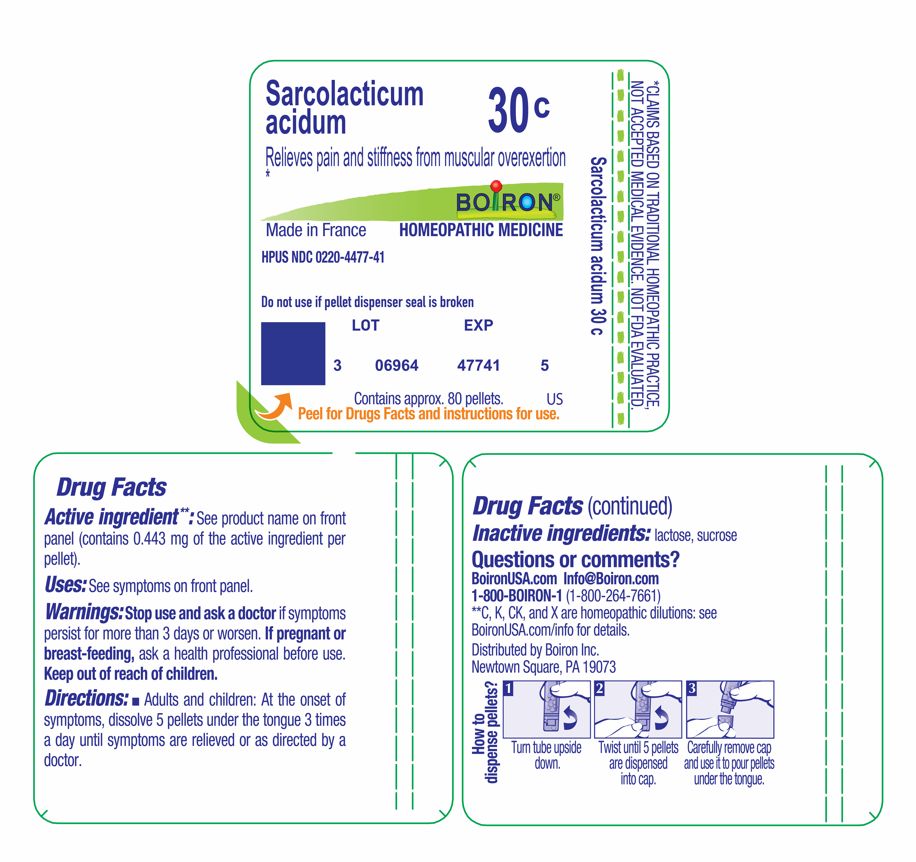
Sarcolacticum acidum 30C
Active ingredient**: See product name on front panel (**contains 0.443 mg of the active ingredient per pellet).
Do not use if pellet dispenser seal is broken.
Contains approx 80 pellets.
How to dispense pellets? Turn tube upside down. Twist until 5 pellets are dispensed into cap. Carefully remove the cap and use it to pour pellets under the tongue.
*CLAIMS BASED ON TRADITIONAL HOMEOPATHIC PRACTICE NOT ACCEPTED MEDICAL EVIDENCE. NOT FDA EVALUATED.
**C,K,CK, and X are homeopathic dilutions: see BoironUSA.com/info for details.
Adults and children: At the onset of symptoms, dissolve 5 pellets under the tongue 3 times a day until symptoms are relieved or as directed by a doctor.