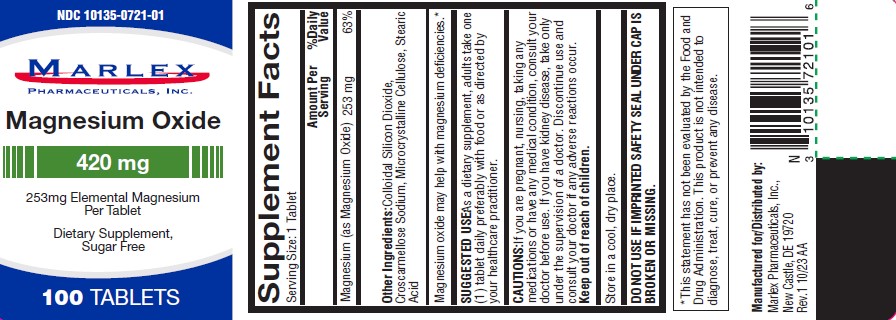
Supplement Facts
|
Serving Size |
1 Tablet |
|
Magnesium (as Magnesium Oxide) |
|
|
Amount per Serving |
253mg |
|
% Daily value |
63% |
Other Ingredients
Colloidal silicon dioxide, Croscarmellose sodium, microcrystalline cellulose, stearic acid
Magnesium oxide may help with magnesium deficiencies.*
SUGGESTED USE
As a dietary supplements, adult take one (1) tablet daily preferably with food or as directed by your healthcare practitioner.
CAUTIONS
If you are pregnant, nursing, taking any medications or have any medical condition, consult your doctor before use. If you have any kidney disease, take only under the supervision of a doctor. Discontinue use and consult your doctor if any adverse reactions occur.