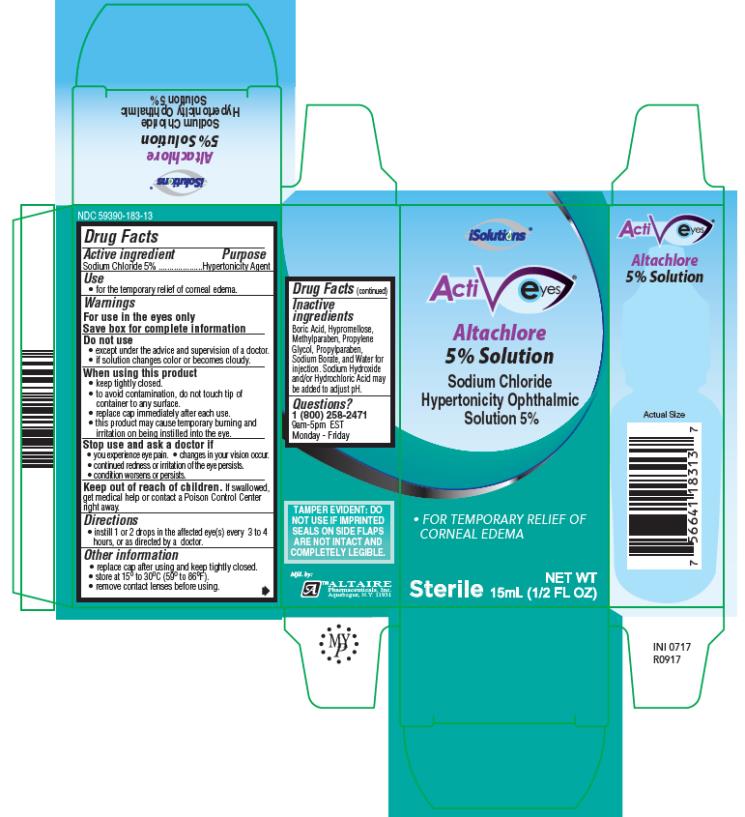
iSolutions
ActivEyes
Altachlore5% Solution
Sodium Chloride Hypertonicity
OphthalmicSolution5%
15mL
NDC 59390-183-13
Drug Facts
Warnings
For use in the eyesonly
Save box for complete information
Do not use
- except under the advice and supervision of a doctor.
- if solution changes color or becomes cloudy.
When using this product
- keep tightly closed.
- to avoid contamination, do not touch tip of container to any surface.
- replace cap immediately after each use.
- this product may cause temporary burning and irritation on being instilled into the eye.
Directions
- instill 1 or 2 drops in the affected eye(s) every 3 to 4 hours, or as directed by a doctor.
Other information
- replace cap after using and keep tightly closed.
- store at 15° to 30°C (59° to 86°F).
- remove contact lenses before using.