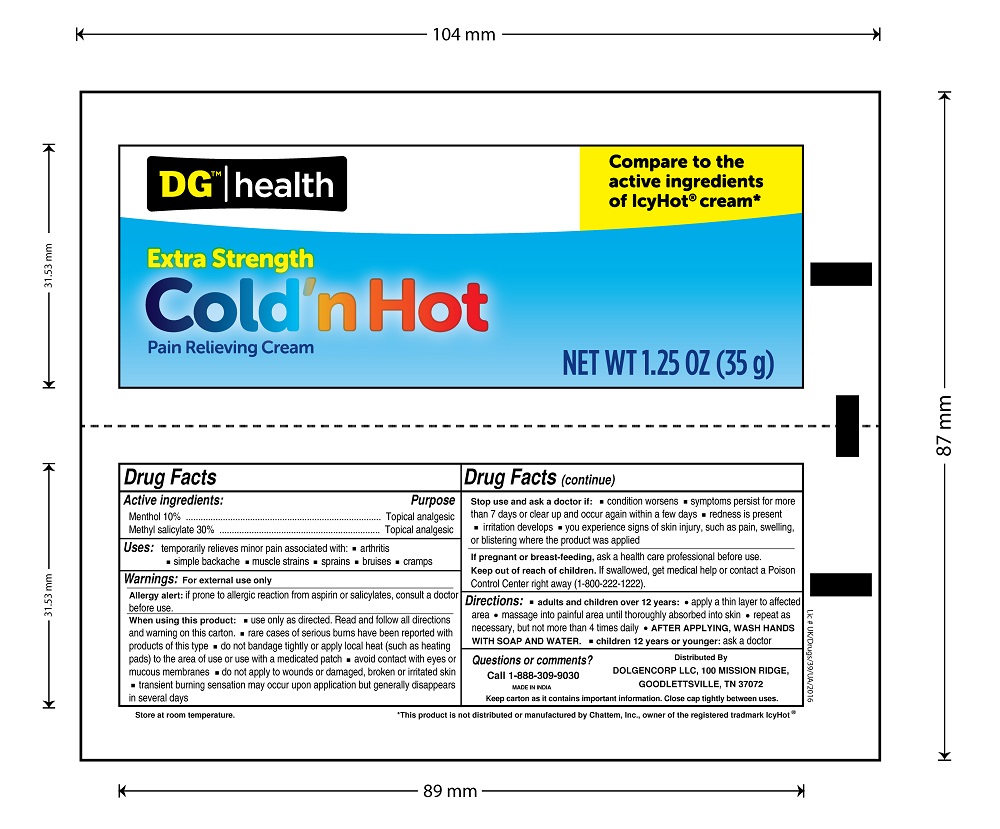
Uses
temporarily relieves minor pain associated with:
- arthritis
- simple backache
- muscle strains
- sprains
- bruises
- cramps
Warnings
For external use only
SEE INSIDE LABEL FOR COMPLETE DRUG FACTS
Allergy Alert: If prone to allergic reaction from aspirin or salicylates, consult a doctor before use.
When using this product
- use only as directed
- do not bandage tightly or use with a heating pad
- avoid contact with eyes or mucous membranes
- do not apply to wounds or damaged, broken or irritated skin
Directions
adults and children over 12 years:
- apply generously to affected area
- massage into painful area until thoroughly absorbed into skin
- repeat as necessary, but not more than 4 times daily
children 12 years or younger: ask a doctor