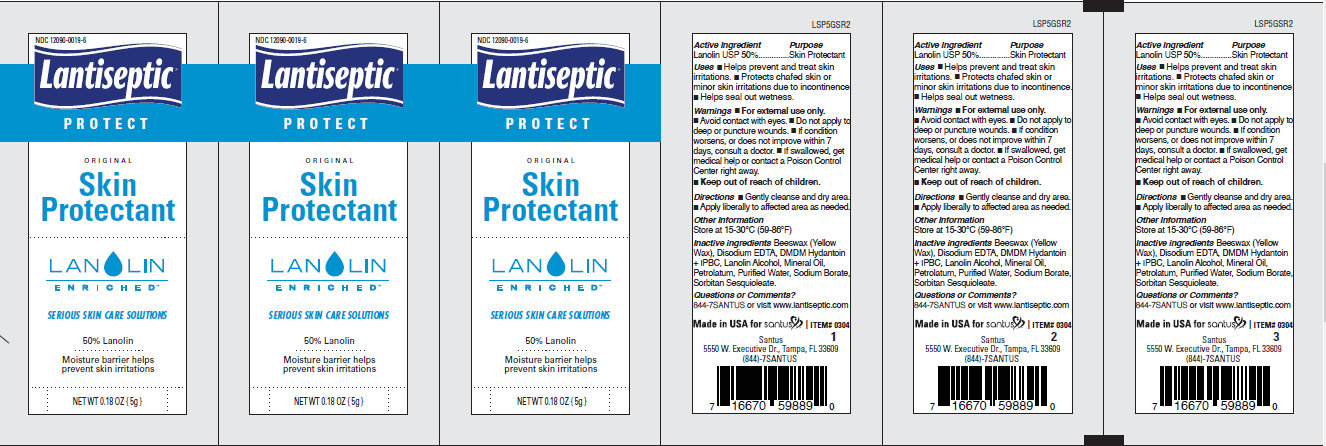
LANTISEPTIC ORIGINAL SKIN PROTECTANT- lanolin cream
Santus LLC
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Lantiseptic Original Skin Protectant Cream
Uses
• Helps prevent and treat skin irritations. • Protects chafed skin or minor skin irritations due to incontinence •Helps seal out wetness.
Warnings
• For external use only. • Avoid contact with eyes. • Do not apply to deep or puncture wounds. • If condition worsens, or does not improve within 7 days, consult a doctor. • If swallowed, get medical help or contact a Poison Control Center right away.
| LANTISEPTIC ORIGINAL SKIN PROTECTANT
lanolin cream |
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
| Labeler - Santus LLC (079868223) |
Revised: 1/2020
Document Id: 9d4b5967-cb24-0e61-e053-2a95a90a5c0b
Set id: daefbb0e-7650-4f35-b337-14a10f869ae2
Version: 5
Effective Time: 20200129
Santus LLC