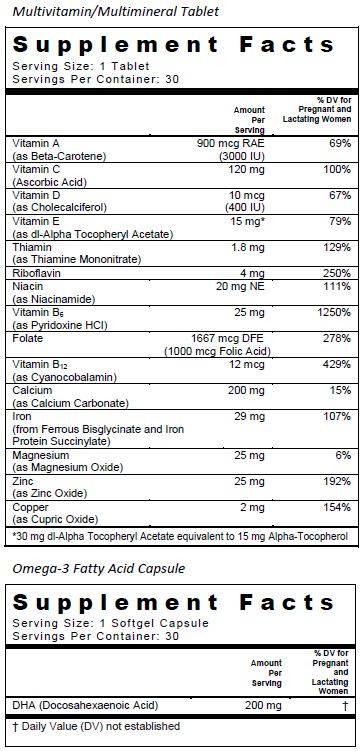
SUPPLEMENT FACTS
Other Ingredients:
Tablets: Microcrystalline Cellulose, Croscarmellose Sodium, Tripotassium Citrate, Coating (Hypromellose, Polyethylene Glycol, Polysorbate 80, Castor Oil), Acacia, Citric Acid, Povidone K30, Fumed Silica, Magnesium Stearate and Stearic Acid
Soft gelatin capsules: Bovine Gelatin, Glycerin, Water, and Ethyl Vanillin.
THIS PRODUCT CONTAINS FISH OIL (ANCHOVIES & SARDINES).
Complete Natal DHA is a multivitamin/multimineral and omega-3 fatty acid supplement indicated for use in improving the nutritional status of women prior to conception, throughout pregnancy, and in the post-natal period for both lactating and non-lactating mothers.
CONTRAINDICATIONS
Supplemental vitamins and minerals should not be prescribed for patients with hemochromatosis or Wilson’s disease. This product is contraindicated in patients with known hypersensitivity to any of the ingredients including fish oil.
WARNINGS
Administration of omega-3 fatty acids should be avoided in patients taking anticoagulants and in those known to have an inherited or acquired predisposition to bleeding.
|
WARNING: Accidental overdose of iron-containing products is a leading cause of fatal poisoning in children under 6. KEEP THIS PRODUCT OUT OF REACH OF CHILDREN. In case of accidental overdose, call a doctor or poison control center immediately. |
PRECAUTIONS
General: Folic acid alone is improper therapy in the treatment of pernicious anemia and other megaloblastic anemias where vitamin B12 is deficient. Folic acid in doses above 0.1 mg daily may obscure pernicious anemia in that hematologic remission can occur while neurological manifestations remain progressive. Allergic sensitization has been reported following both oral and parenteral administration of folic acid. The patient’s medical conditions and consumption of other drugs, herbs, and/or supplements should be considered.
Pediatric Use: Safety and effectiveness in pediatric patients have not been established.
Geriatric Use: Safety and effectiveness in elderly populations have not been established.
DRUG INTERACTIONS
Complete Natal DHA is not recommended for and should not be given to patients receiving levodopa because the action of levodopa is antagonized by pyridoxine. There is a possibility of increased bleeding due to pyridoxine interaction with anticoagulants (e.g., Aspirin, Heparin, Clopidogrel).
ADVERSE REACTIONS
Adverse reactions with iron therapy are usually transient and may include constipation, diarrhea, nausea, vomiting, dark stool and abdominal pain.
DESCRIPTION
Complete Natal DHA tablets are clear coated, speckled light brown, oval tablets with debossed "01030" on one side. Complete Natal DHA soft gelatin capsules are oblong, clear transparent soft gel capsules containing a pale yellow oil/liquid.
DIRECTIONS FOR USE
Take one tablet and one soft gelatin capsule by mouth daily, or as prescribed by a physician. The tablet and soft gelatin capsule may be taken together or at different times of the day.
HOW SUPPLIED
Complete Natal DHA is supplied in a 30 day unit-of-use dispensing carton containing 6 blister cards of 5 tablets and 5 soft gelatin capsules each.
Product Code: 13811-010-30
STORAGE
Store at 20°-25°C (68°-77°F), excursions permitted to 15°-30°C (59°-86°F) [See USP Controlled Room Temperature]. Protect from moisture and excessive heat. Note that contact with moisture may produce surface discoloration of the tablet.
KEEP OUT OF REACH OF CHILDREN.
For use on the order of a healthcare practitioner.
Call your doctor about side effects. To report side effects, call Trigen Laboratories, LLC at 1-770-509-4500 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
PLR-COMNDHA-00001-1 Rev. 01/2022
Manufactured for:
Trigen Laboratories, LLC
Alpharetta, GA 30005
www.trigenlab.com

