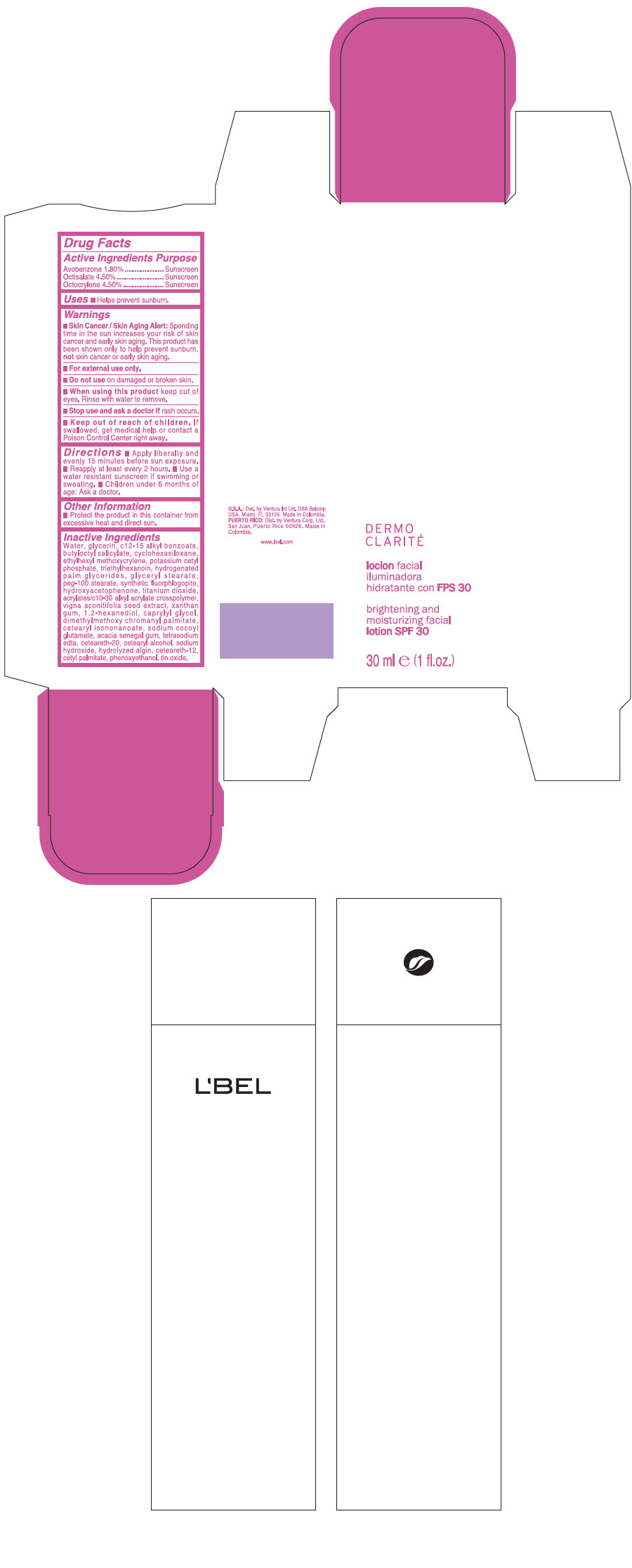
LBEL DERMO CLARITE BRIGHTENING AND MOISTURIZING FACIAL SPF 30- avobenzone, octisalate, and octocrylene lotion
Ventura Corporation LTD
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
| Active Ingredients | Purpose |
| Avobenzone 1.80 % | Sunscreen |
| Octisalate 4.50 % | Sunscreen |
| Octocrylene 4.50 % | Sunscreen |
Warnings
-
Skin Cancer / Skin Aging Alert: Spending time in the sun increases your risk of skin cancer and early skin aging. This product has been shown only to help prevent sunburn, not skin cancer or early skin aging.
-
For external use only.
-
Do not use on damaged or broken skin.
-
When using this product keep out of eyes. Rinse with water to remove.
-
Stop use and ask a doctor if rash occurs.
-
Keep out of reach of children. If swallowed, get medical help or contact a Poison Control Center right away.
Directions
- Apply liberally and evenly 15 minutes before sun exposure.
- Reapply at least every 2 hours
- Use a water resistant sunscreen if swimming or sweating.
- Children under 6 months of age: Ask a doctor
Other information
- Protect the product in this container from excessive heat and direct sun.
Inactive ingredients
WATER, GLYCERIN, C12-15 ALKYL BENZOATE, BUTYLOCTYL SALICYLATE, CYCLOHEXASILOXANE, ETHYLHEXYL METHOXYCRYLENE, POTASSIUM CETYL PHOSPHATE, TRIETHYLHEXANOIN, HYDROGENATED PALM GLYCERIDES, GLYCERYL STEARATE, PEG-100 STEARATE, SYNTHETIC FLUORPHLOGOPITE, HYDROXYACETOPHENONE, TITANIUM DIOXIDE, ACRYLATES/C10-30 ALKYL ACRYLATE CROSSPOLYMER, VIGNA ACONITIFOLIA SEED EXTRACT, XANTHAN GUM, 1,2-HEXANEDIOL, CAPRYLYL GLYCOL, DIMETHYLMETHOXY CHROMANYL PALMITATE, CETEARYL ISONONANOATE, SODIUM COCOYL GLUTAMATE, ACACIA SENEGAL GUM, TETRASODIUM EDTA, CETEARETH-20, CETEARYL ALCOHOL, SODIUM HYDROXIDE, HYDROLYZED ALGIN, CETEARETH-12, CETYL PALMITATE, PHENOXYETHANOL, TIN OXIDE
Dist. by Ventura Corp Ltd.,
San Juan, Puerto Rico 00926.
PRINCIPAL DISPLAY PANEL - 30 ml Jar Carton
L'BEL
DERMO
CLARITÉ
brightening and
moisturizing facial
lotion SPF 30
30 ml e (1 fl.oz.)