Uses
- helps prevent sunburn
- if used as directed with other sun protection measures (see Directions), decreases the risk of skin cancer and early skin aging caused by the sun
Directions
For sunscreen use:
- apply liberally 15 minutes before sun exposure
- use a water resistant sunscreen if swimming or sweating
- reapply at least every 2 hours
-
Sun Protection Measures. Spending time in the sun increases your risk of skin cancer and early skin aging. To decrease this risk, regularly use a sunscreen with a broad spectrum SPF of 15 or higher and other sun protection measures including:
- limit time in the sun, especially from 10 a.m. – 2 p.m.
- wear long-sleeve shirts, pants, hats, and sunglasses
- children under 6 months: Ask a doctor
Inactive Ingredients
TALC•SYNTHETIC FLUORPHLOGOPITE•BARIUM SULFATE•ZINC OXIDE•TRIETHYLHEXANOIN•DIMETHICONE•SILICA•NYLON-12•STEAROXYMETHICONE/DIMETHICONE COPOLYMER•VINYL DIMETHICONE/METHICONE SILSESQUIOXANE CROSSPOLYMER•CALCIUM ALUMINUM BOROSILICATE•DIPHENYL DIMETHICONE/VINYL DIPHENYL DIMETHICONE/SILSESQUIOXANE CROSSPOLYMER•DIPHENYLSILOXY PHENYL TRIMETHICONE•HYDROGENATED POLYISOBUTENE•ROSA ROXBURGHII FRUIT EXTRACT•DIMETHICONE/VINYL DIMETHICONE CROSSPOLYMER•SODIUM ACETYLATED HYALURONATE•SORBITAN SESQUIISOSTEARATE•ALUMINUM HYDROXIDE•TRIETHOXYSILYLETHYL POLYDIMETHYLSILOXYETHYL HEXYL DIMETHICONE•ALUMINUM DISTEARATE•METHICONE•TRIETHOXYSILYLETHYL POLYDIMETHYLSILOXYETHYL DIMETHICONE•SODIUM MAGNESIUM SILICATE•POLYSILICONE-2•MAGNESIUM MYRISTATE•BUTYLENE GLYCOL•WATER•GLYCERIN•TOCOPHEROL•DIMETHICONE/METHICONE COPOLYMER•HYDROGEN DIMETHICONE•BHT•POLYMETHYLSILSESQUIOXANE•ALUMINA•CHLORPHENESIN•FRAGRANCE•MICA•TITANIUM DIOXIDE•BISMUTH OXYCHLORIDE•IRON OXIDES•
PRINCIPAL DISPLAY PANEL - 11 g Tray Carton - I10
clé de peau
BEAUTÉ
radiant powder foundation
BROAD SPECTRUM SPF 23
SUNSCREEN
11g NET WT. .38 OZ.

PRINCIPAL DISPLAY PANEL - 11 g Tray Carton - O10
clé de peau
BEAUTÉ
radiant powder foundation
BROAD SPECTRUM SPF 23
SUNSCREEN
11g NET WT. .38 OZ.
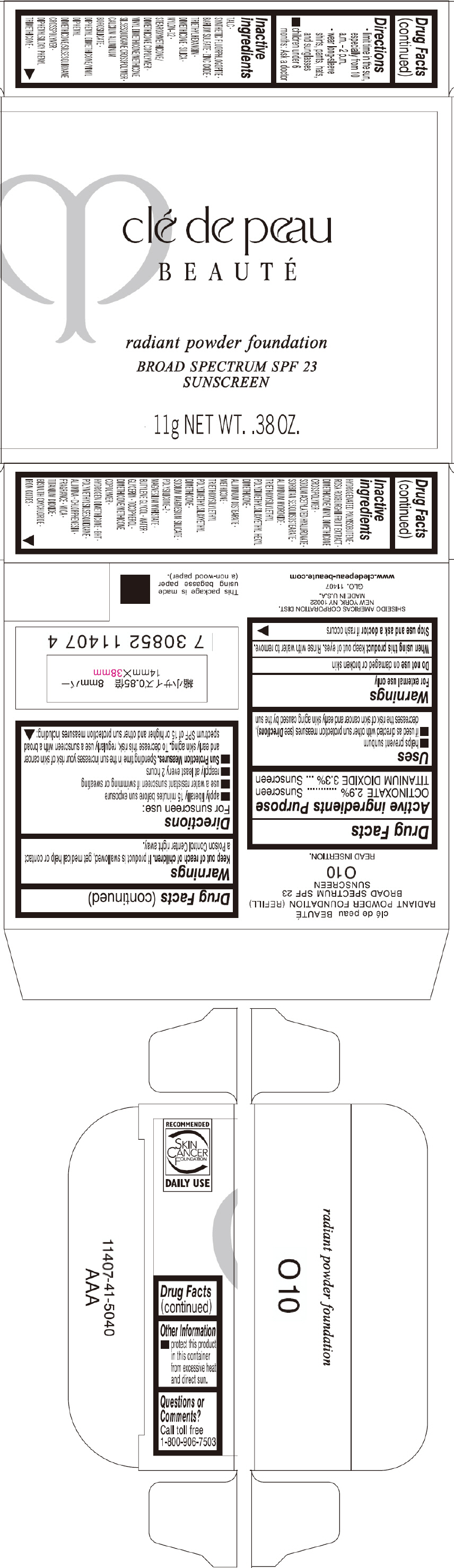
PRINCIPAL DISPLAY PANEL - 11 g Tray Carton - O20
clé de peau
BEAUTÉ
radiant powder foundation
BROAD SPECTRUM SPF 23
SUNSCREEN
11g NET WT. .38 OZ.
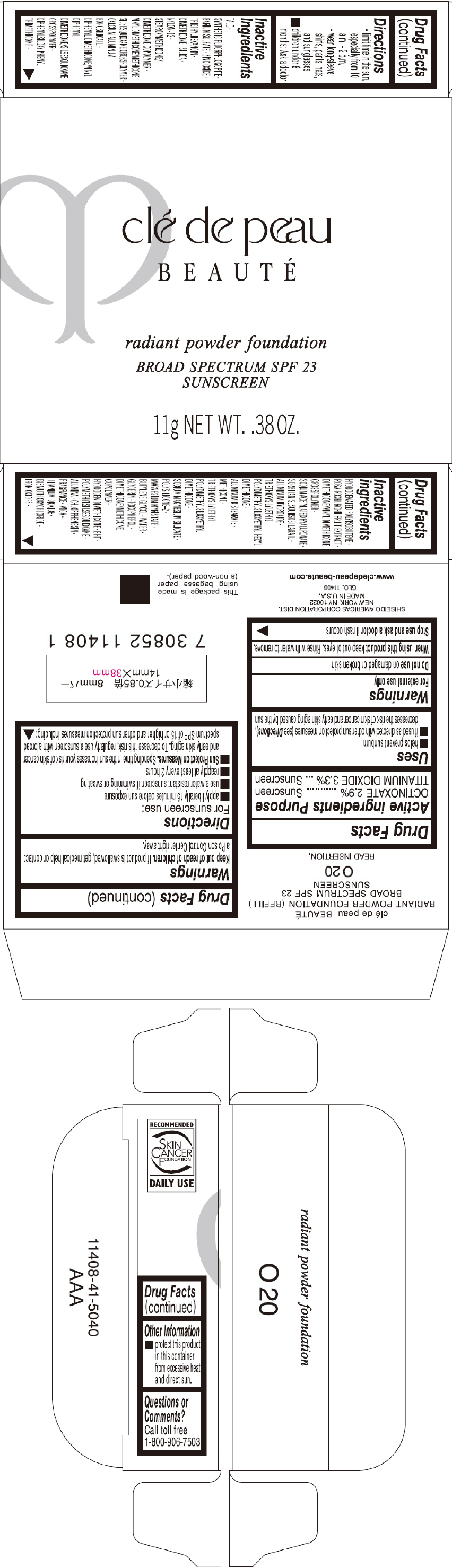
PRINCIPAL DISPLAY PANEL - 11 g Tray Carton - O30
clé de peau
BEAUTÉ
radiant powder foundation
BROAD SPECTRUM SPF 23
SUNSCREEN
11g NET WT. .38 OZ.
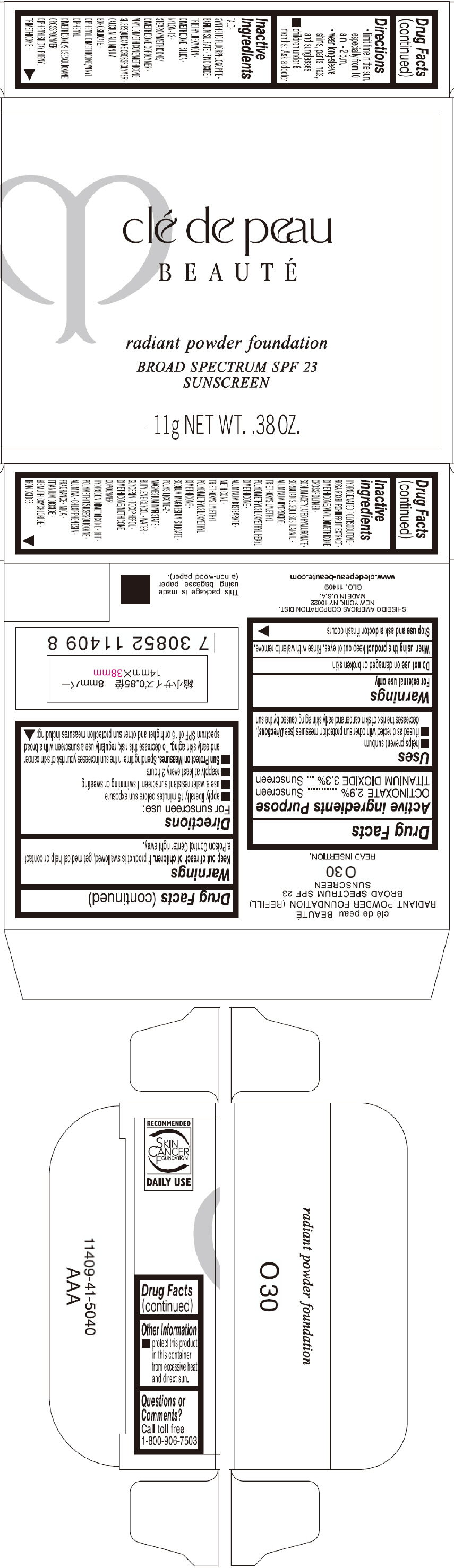
PRINCIPAL DISPLAY PANEL - 11 g Tray Carton - O40
clé de peau
BEAUTÉ
radiant powder foundation
BROAD SPECTRUM SPF 23
SUNSCREEN
11g NET WT. .38 OZ.
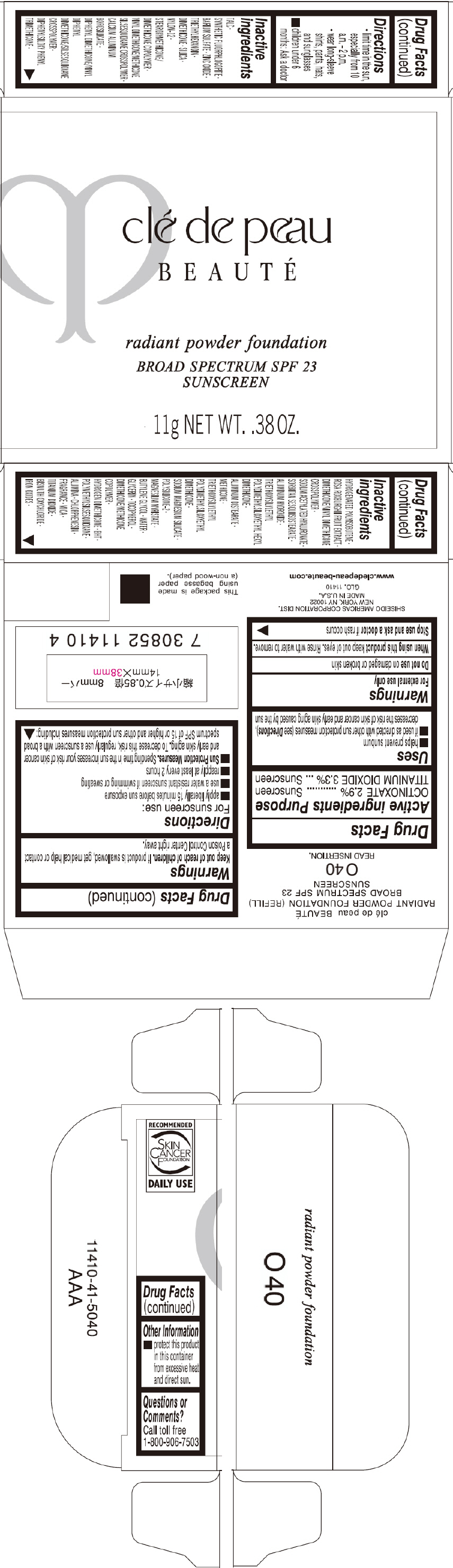
PRINCIPAL DISPLAY PANEL - 11 g Tray Carton - O50
clé de peau
BEAUTÉ
radiant powder foundation
BROAD SPECTRUM SPF 23
SUNSCREEN
11g NET WT. .38 OZ.

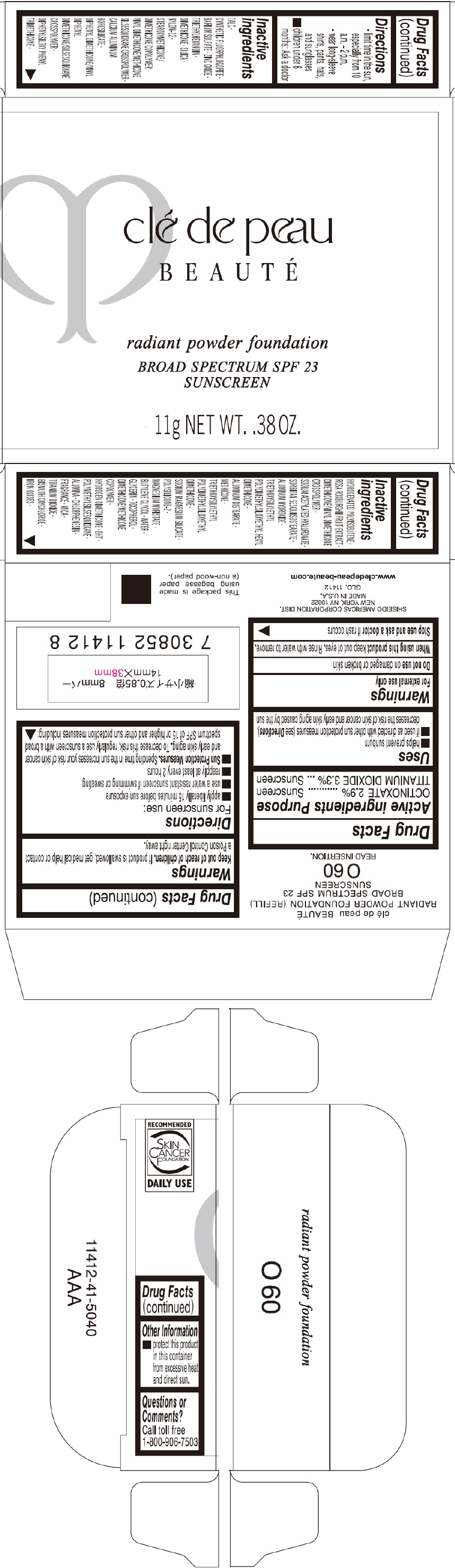
PRINCIPAL DISPLAY PANEL - 11 g Tray Carton - O60
clé de peau
BEAUTÉ
radiant powder foundation
BROAD SPECTRUM SPF 23
SUNSCREEN
11g NET WT. .38 OZ.
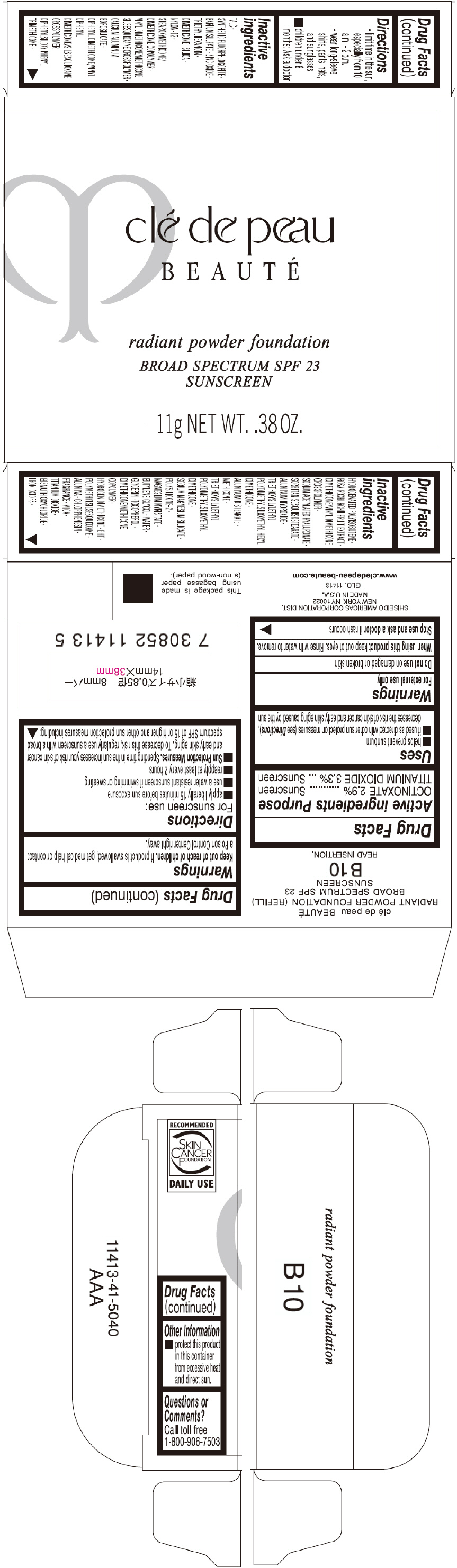
PRINCIPAL DISPLAY PANEL - 11 g Tray Carton - B10
clé de peau
BEAUTÉ
radiant powder foundation
BROAD SPECTRUM SPF 23
SUNSCREEN
11g NET WT. .38 OZ.
PRINCIPAL DISPLAY PANEL - 11 g Tray Carton - B20
clé de peau
BEAUTÉ
radiant powder foundation
BROAD SPECTRUM SPF 23
SUNSCREEN
11g NET WT. .38 OZ.

PRINCIPAL DISPLAY PANEL - 11 g Tray Carton - B30
clé de peau
BEAUTÉ
radiant powder foundation
BROAD SPECTRUM SPF 23
SUNSCREEN
11g NET WT. .38 OZ.


