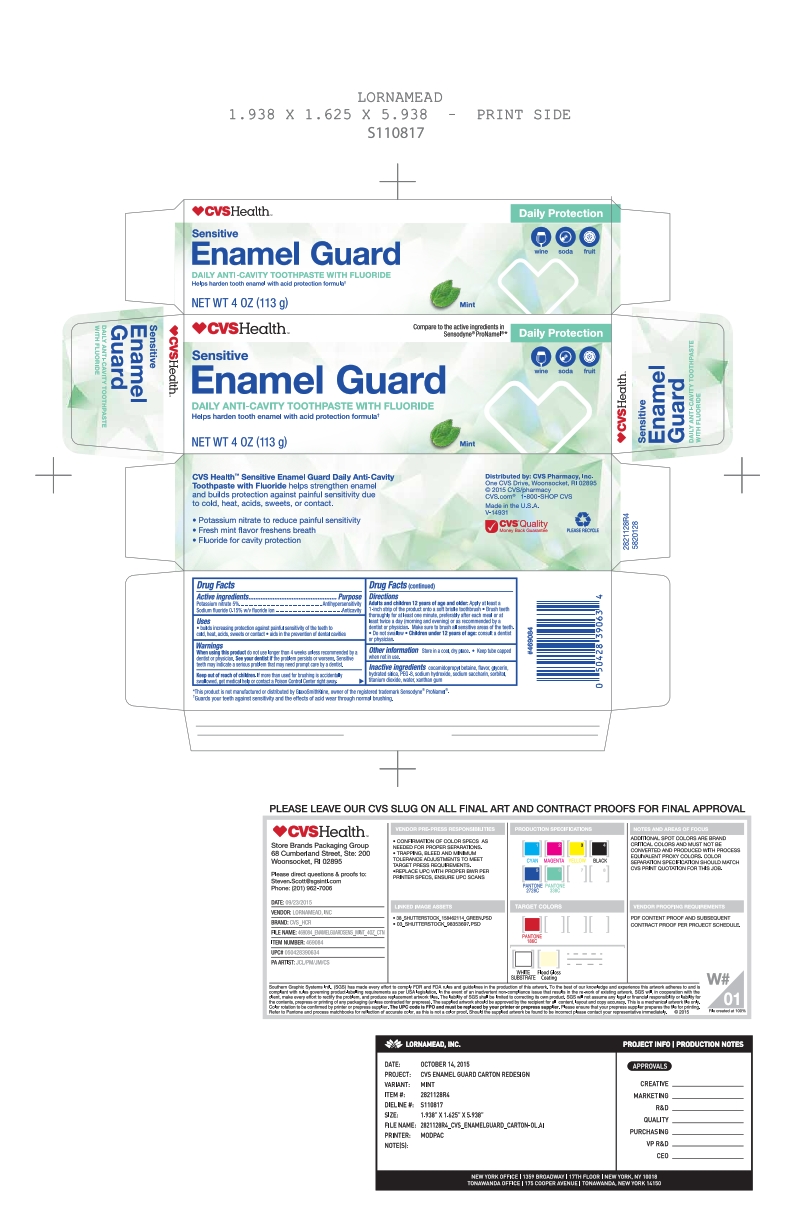
Active ingredients / Purpose
Potassium nitrate 5%....................................................Antihypersensitivity
Sodium fluoride 0.15% w/v fluoride ion.........................Anticavity
When using this product do not use longer than 4 weeks unless recommended by a dentist or physician. See your dentist if the problem worsens. Sensitive teeth may indicate a serious problem that may need prompt care by a dentist.
Directions
Brush teeth thoroughly for at least one minute, preferably after each meal or at least twice a day (morning and evening) or as recommended by a dentist or physican. Make sure to brush all sensitive areas of the teeth. Do not swallow.
Apply at least a 1-inch strip of the product onto a soft bristle toothbrush. Children under 12 years of age: consult a dentist or a doctor.