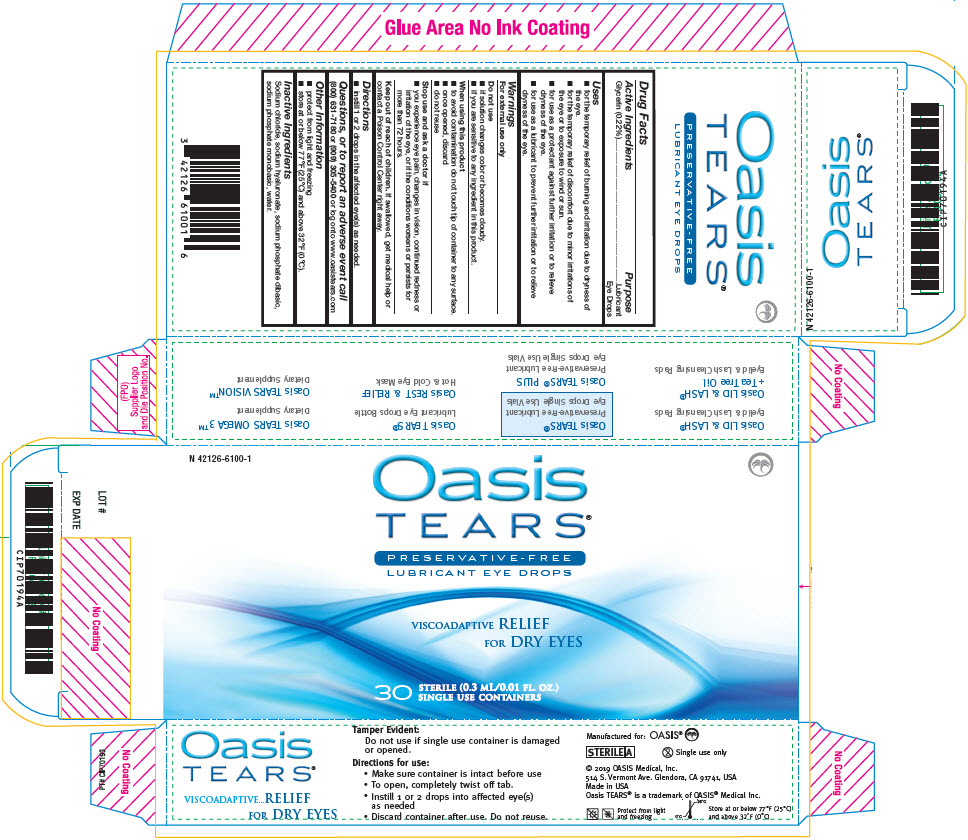
OASIS TEARS PRESERVATIVE-FREE LUBRICANT EYE- glycerin solution/ drops
OASIS Medical, Inc.
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Active Ingredients
Glycerin (0.22%)
Purpose
Lubricant Eye Drops
Uses
- for the temporary relief of burning and irritation due to dryness of the eye.
- for the temporary relief of discomfort due to minor irritations of the eye or to exposure to wind or sun.
- for use as a protectant against further irritation or to relieve dryness of the eye.
- for use as a lubricant to prevent further irritation or to relieve dryness of the eye.
Warnings
For external use only
Do not use
- if solution changes color or becomes cloudy.
- if you are sensitive to any ingredient in this product.
When using this product
- to avoid contamination do not touch tip of container to any surface.
- once opened, discard
- do not reuse
Stop use and ask a doctor if
- you experience eye pain, changes in vision, continued redness or irritation of the eye, or if the conditions worsens or persists for more than 72 hours.
Keep out of reach of children. If swallowed, get medical help or contact a Poison Control Center right away.
Directions
- instill 1 or 2 drops in the affected eye(s) as needed.
Questions, or to report an adverse event call
(800) 631-7180 or (909) 305-5400 or log onto www.oasistears.com
Other Information
- protect from light and freezing
- store at or below 77°F (25°C) and above 32°F (0°C).
Inactive Ingredients
Sodium chloride, sodium hyaluronate, sodium phosphate dibasic, sodium phosphate monobasic, water.
PRINCIPAL DISPLAY PANEL - 0.3 mL Pouch Carton
N 42126-6100-1
Oasis
TEARS®
PRESERVATIVE - FREE
LUBRICANT EYE DROPS
VISCOADAPTIVE RELIEF
FOR DRY EYES
30
STERILE (0.3 mL/0.01 FL. OZ.)
SINGLE USE CONTAINERS
OASIS Medical, Inc.