SUPPLEMENT FACTS
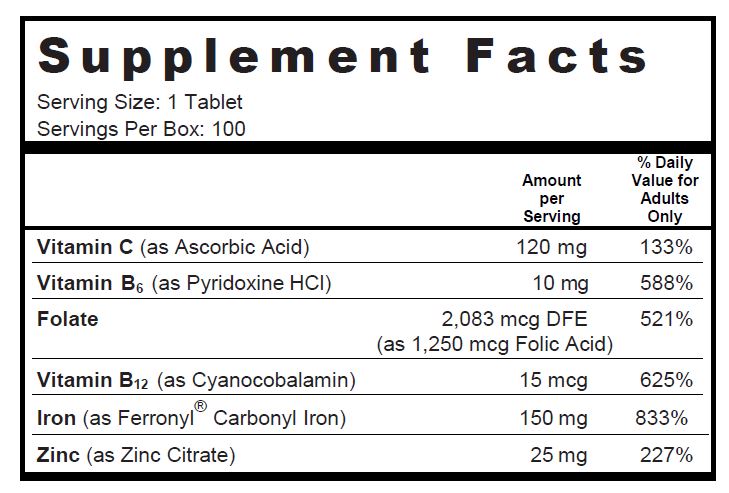
Other Ingredients: Microcrystalline Cellulose, Pregelatinized Starch, Red Color Coating (Polyvinyl Alcohol, Polyethylene Glycol, FD&C Red #40 Lake, Talc, Titanium Dioxide), Croscarmellose Sodium, Magnesium Stearate, Stearic Acid
GLUTEN-, LACTOSE- AND SUGAR-FREE
CORVITA™ 150 is a prescription multivitamin/multimineral supplement used to improve the nutritional status of patients with iron deficiency
CONTRAINDICATIONS
CORVITA™ 150 should not be used by patients with a known hypersensitivity to any of the listed ingredients. All iron compounds are contraindicated in patients with hemochromatosis, hemosiderosis, or hemolytic anemias.
| WARNING: Accidental overdose of iron-containing products is a leading cause of fatal poisoning in children under 6. KEEP THIS PRODUCT OUT OF REACH OF CHILDREN. In case of accidental overdose, call a doctor or poison control center immediately. |
PRECAUTIONS
General: Do not exceed recommended dose. The type of anemia and the underlying cause or causes should be determined before starting therapy with CORVITA™150. Since the anemia may be a result of a systemic disturbance, such as recurrent blood loss, the underlying cause or causes should be corrected, if possible.
Folic Acid: Folic acid alone is an improper therapy in the treatment of pernicious anemia and other megaloblastic anemias where vitamin B12 is deficient. Folic acid in doses above 0.1 mg daily may obscure pernicious anemia in that hematologic remission can occur while neurologic manifestations remain progressive. Allergic sensitization has been reported following both oral and parenteral administration of folic acid.
The patient’s medical conditions and consumption of other drugs, herbs, and/or supplements should be considered.
Pediatric Use: Safety and effectiveness in pediatric populations have not been established.
Geriatric Use: Safety and effectiveness in elderly populations have not been established.
DRUG INTERACTIONS
CORVITA™150 is not recommended for and should not be given to patients receiving levodopa because the action of levodopa is antagonized by pyridoxine. There is a possibility of increased bleeding due to pyridoxine interaction with anticoagulants (e.g., Aspirin, Heparin, Clopidogrel).
ADVERSE REACTIONS
Adverse reactions with iron therapy may include constipation, diarrhea, nausea, vomiting, dark stools and abdominal pain. Adverse reactions with iron therapy are usually transient.
OVERDOSAGE
The clinical course of acute iron overdosage can be variable. Initial symptoms may include abdominal pain, nausea, vomiting, diarrhea, tarry stools, melena, hematemesis, hypotension, tachycardia, metabolic acidosis, hyperglycemia, dehydration, drowsiness, pallor, cyanosis, lassitude, seizures, shock and coma.
HOW SUPPLIED
CORVITA™ 150 tablets are supplied in unit dose packs of 100 (10 blister cards of 10 tablets each).
PRODUCT CODE 13811-066-10
STORAGE
Store at 20°-25°C (68°-77°F), excursions permitted to 15°-30°C (59°-86°F) [See USP Controlled Room Temperature]. Protect from light and moisture and avoid excessive heat. Dispense in a tight, light resistant container as defined in the USP using a child-resistant closure.
KEEP THIS PRODUCT OUT OF REACH OF CHILDREN.
For use on the order of a healthcare practitioner
Call your doctor about side effects. To report side effects, call Trigen Laboratories at 1-770-509-4500 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
PLR-CORVITA150-00001-1 Rev. 12/2021
Manufactured for:
Trigen Laboratories, LLC
Alpharetta, GA 30005
www.trigenlab.com