DESCRIPTION
Sterile Water for Irrigation USP is a sterile, hypotonic, nonpyrogenic irrigating fluid or pharmaceutic aid (solvent) entirely composed of Sterile Water for Injection USP. It is prepared by distillation and contains no antimicrobial or bacteriostatic agents or added buffers.
The pH is 5.7 (5.0-7.0)
The plastic container is made from a multilayered film specifically developed for parenteral drugs. It contains no plasticizers and exhibits virtually no leachables. The solution contact layer is a rubberized copolymer of ethylene and propylene. The container is nontoxic and biologically inert. The container-solution unit is a closed system and is not dependent upon entry of external air during administration. The container is overwrapped to provide protection from the physical environment and to provide an additional moisture barrier when necessary.
Not made with natural rubber latex, PVC or DEHP.
CLINICAL PHARMACOLOGY
Sterile Water for Irrigation USP is utilized for a variety of clinical indications. Because of its low refractive index (1.3325), water provides excellent visibility during endoscopic urological procedures. It is also utilized as a pharmaceutic aid, as well as in the preparation of enteral nutrient products.
Water is hypotonic and will cause hemolysis and will be readily absorbed by the tissues during surgical procedures; therefore, its use under such conditions is not recommended.
INDICATIONS AND USAGE
Sterile Water for Irrigation USP is indicated for use as an irrigating fluid or pharmaceutic aid. Sterile Water may also be used as an adjunct in the preparation of non-intravenously administered nutrient mixtures (see DOSAGE AND ADMINISTRATION).
WARNINGS
Sterile Water for Irrigation USP is hypotonic and will cause hemolysis, and is not recommended for use during surgical procedures.
After opening container, its contents should be used promptly to minimize the possibility of bacterial growth or pyrogen formation.
Discard unused portion of irrigating solution since it contains no preservative.
DOSAGE AND ADMINISTRATION
Irrigation
Use as directed by physician.
This drug product should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit.
Nutrient Mixtures
Sterile Water for Irrigation USP may be used to prepare non-intravenously administered nutrient mixtures. It contains no electrolytes or other added substances. Refer to preparation instructions of particular mixture to be used. The plastic container may be used for administration of non-intravenous nutrient mixture to the patient as appropriate.
HOW SUPPLIED
Sterile Water for Irrigation USP is supplied sterile and nonpyrogenic in single-dose 2000 and 3000 mL flexible irrigation containers packaged 4 per case.
| NDC | REF | SIZE |
| 0264-7386-50 | R8005 | 2000 mL |
| 0264-7386-60 | R8006 | 3000 mL |
Exposure of pharmaceutical products to heat should be minimized. Avoid excessive heat. Protect from freezing. It is recommended that the product be stored at room temperature (25°C); however, brief exposure up to 40°C does not adversely affect the product.
Directions for Use of Plastic Container
Not for injection.
Not for use with pressurized irrigation systems.
Aseptic technique is required.
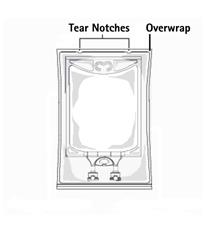
- Inspect irrigation bag: overwrap and primary bag.
- Do not use if overwrap has been damaged.
- Do not use unless solution is clear and closure is intact.
- To open: Tear overwrap starting from the tear notches. (Figure 1)
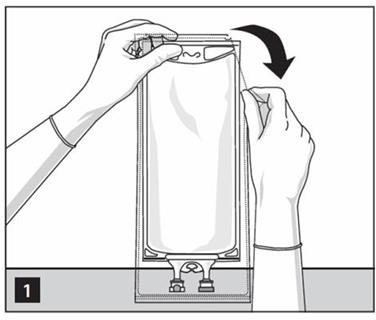
- Prepare medication port by removal of aluminum foil. (Figure 2a)
- Puncture resealable medication port by using 19 – 22 gauge needle and inject additive(s). (Figure 2b)
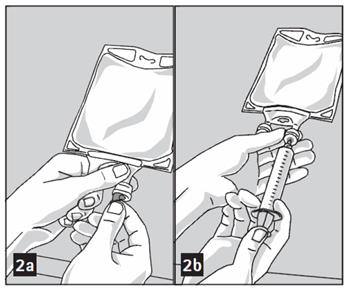
- Mix solution and medication thoroughly. (Figure 3a)
- Medication port must be swabbed with disinfection agent before re-puncturing.
- Check admixture visually for particulate matter. (Figure 3b)
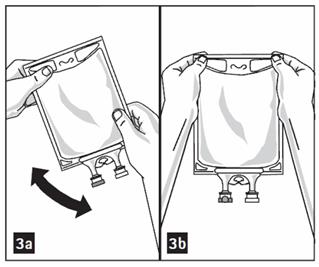
- Puncture resealable medication port by using 19 – 22 gauge needle and inject additive(s). (Figure 2b)
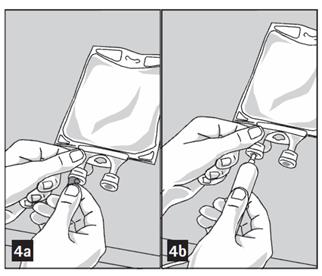
- Remove aluminum foil from outlet/set port at the bottom of container (Figure 4a) and attach administration set (Figure 4b): use non-vented infusion set or close air vent on a vented set. Refer to directions for use accompanying the administration set.
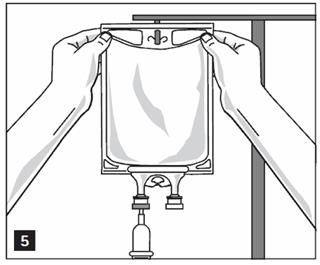
- Hang bag on IV Pole. (Figure 5)
Rx only
B. Braun Medical Inc.
Bethlehem, PA 18018-3524 USA
1-800-227-2862
LD-401-5 Y36-002-984
Revised: August 2018
PRINCIPAL DISPLAY PANEL
STERILE WATERFOR IRRIGATION,
USP
INDICATIONS: FOR IRRIGATION
CONTRAINDICATIONS:
NOT FOR INJECTION
REF R8005
NDC 0264-7386-50
LOT
EXP.
2000 mL
No antimicrobial agent or other
substance has been added.
pH: 5.7 (5.0-7.0)
WARNINGS: HYPOTONIC AND HEMOLYTIC
See Package Insert.
Use only if solution is clear and container and seal
are intact.
Sterile, nonpyrogenic. Single unit container. Discard
unused portion.
Dosage: Irrigation. Use as directed by physician.
Recommended Storage: Room temperature (25°C).
Avoid excessive heat. Protect from freezing.
See Package Insert.
Not made with natural
rubber latex, PVC or DEHP.
Rx only

Y38-000-063 LD-255-6
B. Braun Medical Inc.
Bethlehem, PA 18018-3524 USA
1-800-227-2862
SET


Sterile Water Irrigation 2L Container Label
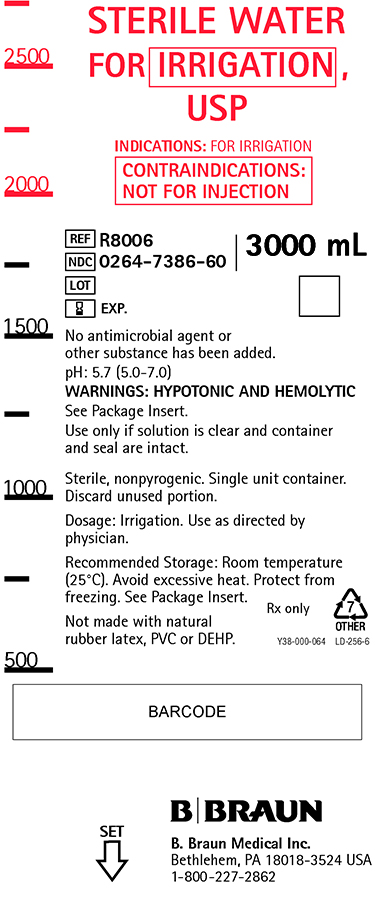
PRINCIPAL DISPLAY PANEL
STERILE WATER
FOR IRRIGATION,
USP
INDICATIONS: FOR IRRIGATION
CONTRAINDICATIONS:
NOT FOR INJECTION
REF R8006
NDC 0264-7386-60
LOT
EXP.
3000 mL
No antimicrobial agent or
other substance has been added.
pH: 5.7 (5.0-7.0)
WARNINGS: HYPOTONIC AND HEMOLYTIC
See Package Insert.
Use only if solution is clear and container
and seal are intact.
Sterile, nonpyrogenic. Single unit container.
Discard unused portion.
Dosage: Irrigation. Use as directed by
physician.
Recommended Storage: Room temperature
(25°C). Avoid excessive heat. Protect from
freezing. See Package Insert.
Not made with natural
rubber latex, PVC or DEHP.
Rx only

Y38-000-064 LD-256-6
B. Braun Medical Inc.
Bethlehem, PA 18018-3524 USA
1-800-227-2862
SET

Sterile Water Irrigation 3L Container Label