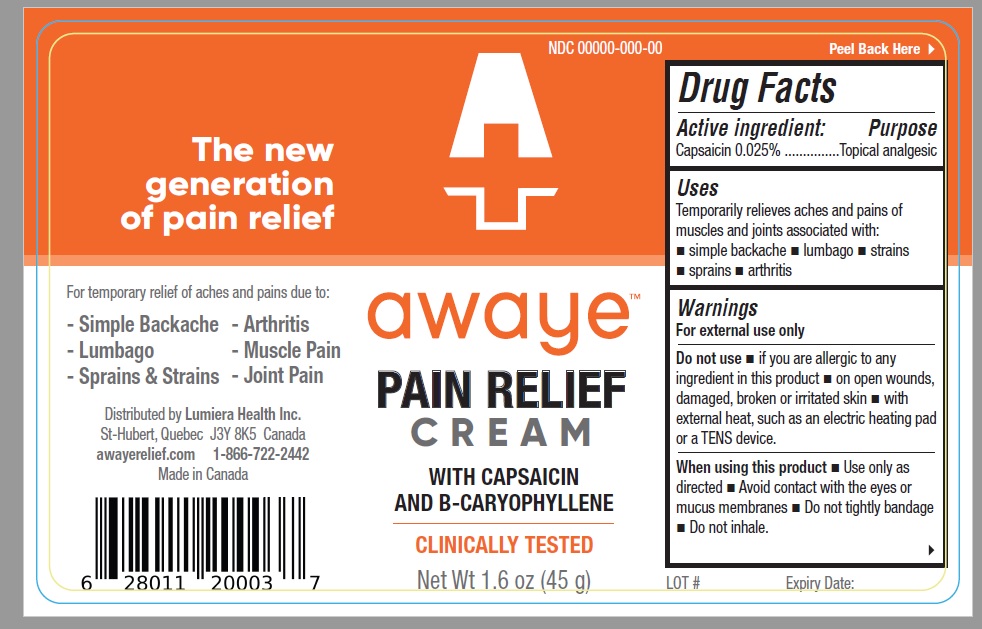
Uses
Temporarily relieves aches and pains of muscles and joints associated with: simple backache – lumbago – strains – sprains - arthritis
Warning
For external use only
Flammable: Keep away from fire or flame and heated surfaces.
Do not use – if you are allergic to any ingredient in this product - on open wounds, damaged, broken or irritated skin - with external heat, such as an electric heating pad or a TENS device.
When using this product – Use only as directed - Avoid contact with the eyes or mucus membranes - Do not tightly bandage - Do not inhale.
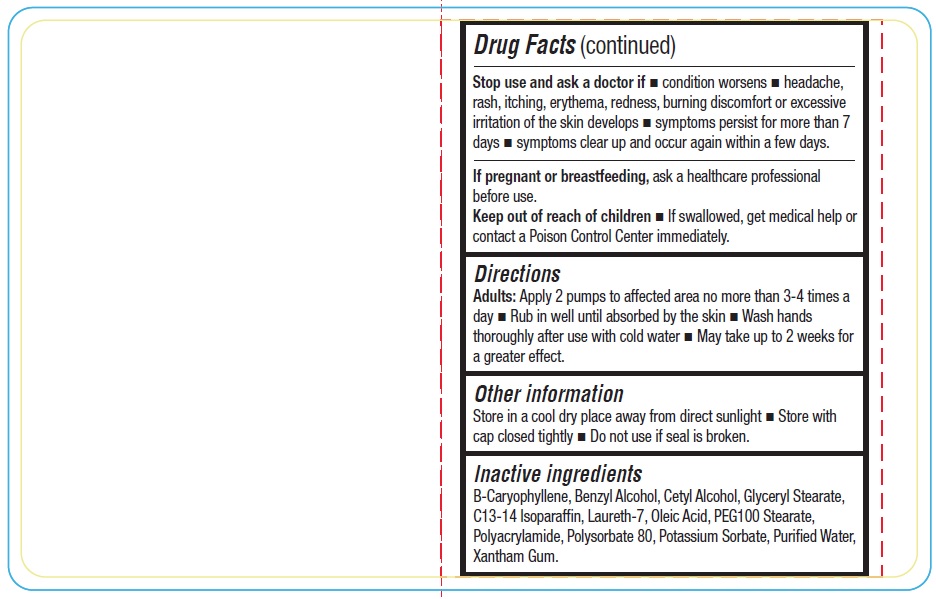
Stop use and ask a doctor if – condition worsens – headache, rash, itching, erythema, redness, burning discomfort or excessive irritation of the skin develops - symptoms persist for more than 7 days – symptoms clear up and occur again within a few days.
If pregnant or breastfeeding, ask a healthcare professional before use.
Keep out of reach of children. If swallowed, get medical help or contact a Poison Control Center immediately.
Direction
Apply 2 pumps to affected area no more than 3-4 times a day - Rub in well until absorbed by the skin - wash hands thoroughly after use with cold water - May take up to 2 weeks for a greater effect.
Inactive ingredient
B-Caryophyllene, Benzyl Alcohol, Cetyl Alcohol, Glyceryl Stearate, C13-14 Isoparaffin, Laureth-7, Oleic Acid, PEG100 Stearate, Polyacrylamide, Polysorbate 80, Potassium Sorbate, Purified Water, Xantham Gum.