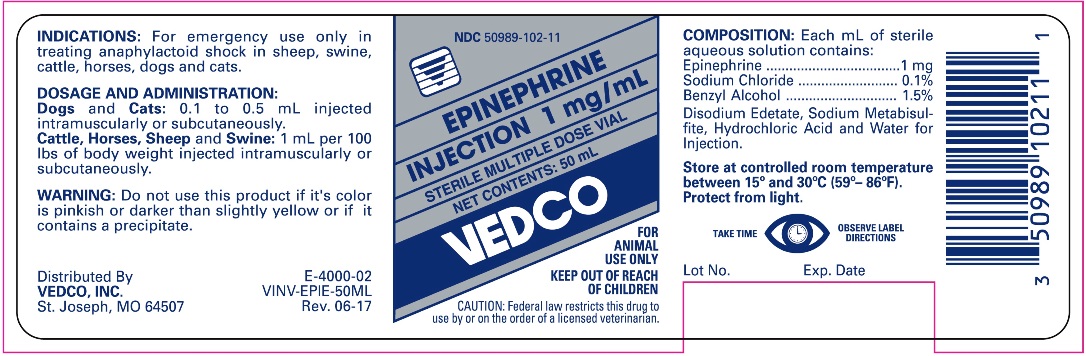
FOR ANIMAL USE ONLY
KEEP OUT OF REACH OF CHILDREN
CAUTION: Federal law restricts this drug to use by or on the order of a licensed veterinarian.
INDICATIONS
For emergency use only in treating anaphylactoid shock in sheep, swine, cattle, horses, dogs and cats.
DOSAGE AND ADMINISTRATION
Dogs and Cats: 0.1 to 0.5 mL injected Intramuscularly or Subcutaneously.
Cattle, Horses, Sheep and Swine: 1 mL per 100 lbs of body weight injected intramuscularly or subcutaneously.
WARNING
Do not use this product if it's color is pinkish or darker than slightly yellow or if it contains a precipitate.
COMPOSITION
Each mL of sterile aqueous solution contains:
Epinephrine ....................... 1 mg
Sodium Chloride ................ 0.1%
Benzyl Alcohol .................. 1.5%
Disodium Edetate, Sodium Metabisulfite, Hydrochloric Acid, and Water for Injection.