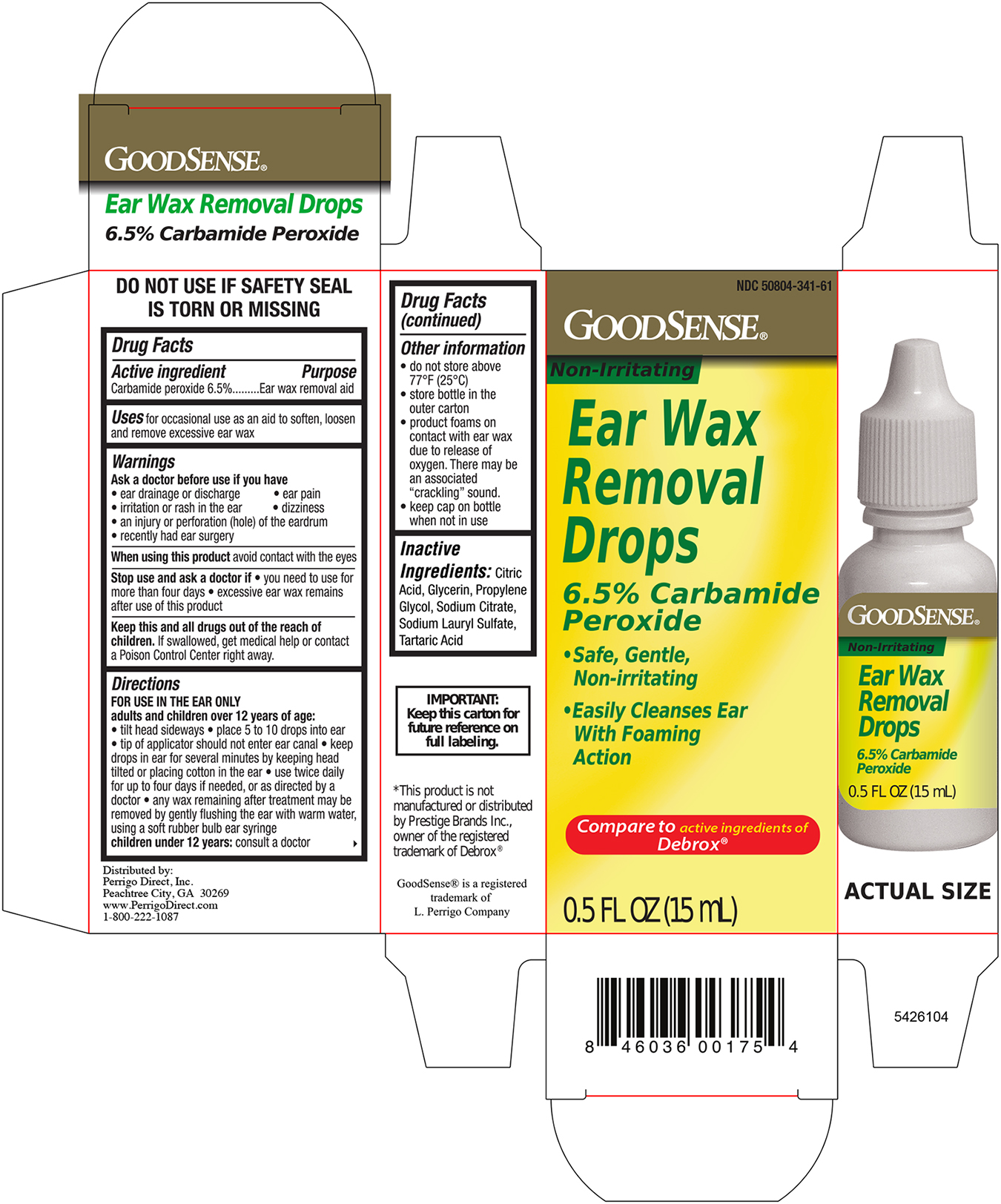
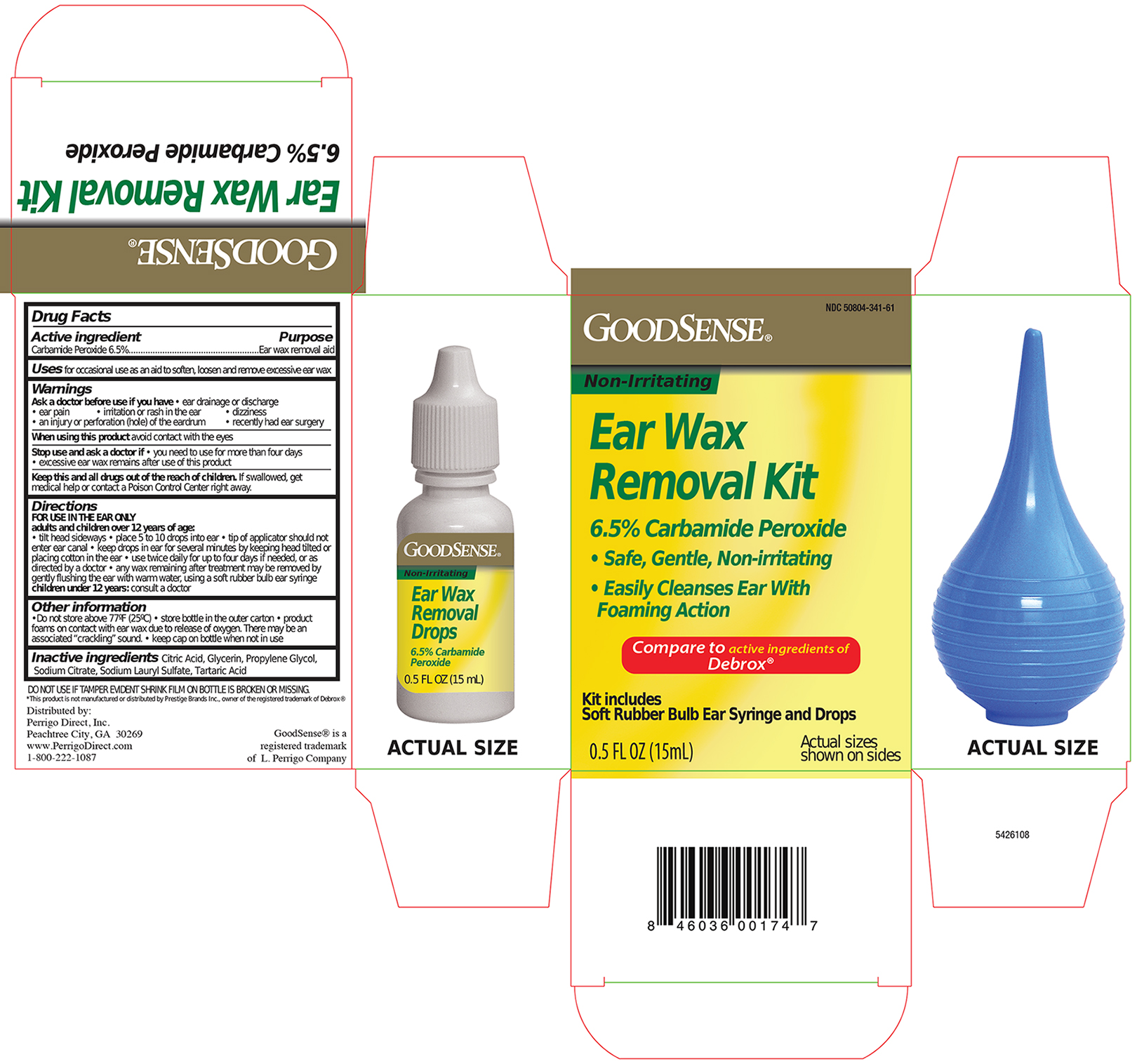
Warnings
Ask a Doctor before use if you have
- eardrainage, discharge, ear pain,irritation
- rash in the ear,or are dizzy
- injury or perforation (hole) of the ear drum
- Recently had ear surgery
Stop Use and ask a Doctor if
- you need to use for more than 4 days
- execessive ear wax remain after use of this product
When using this product
- do not use for more than four days
- avoid contact with the eyes. If accidental contact with the eyes occurs, flush eyes with water and consult a doctor
- if excessive earwax remains after the use of this product, consult a doctor
Keep out of the reach of children
If swallowed, get medical help or contact a Poison Control Center right away.
Directions FOR USE IN THE EAR ONLY
- Adults and children over 12 years of age:
- Tilt head sideways and place 5 to 10 drops into ear.
- Tip of applicator should not enter ear canal.
- Keep drops in ear for several minutes by keeping head tilted or placing cotton in the ear.
- Use twice daily for up to 4 days if needed, or as directed by a doctor.
- Any earwax remaining after treatment may be removed by gently flushing the ear with warm water, using a soft rubber bulb ear syringe.
- When the ear canal is irrigated, the tip of the ear syringe should not obstruct the flow of water leaving the ear canal.
- Children under 12 years: consult a doctor.
Other information
- Protect from heat and direct sunlight
- product foams on contact with ear wax due ot releae of oxygen, there may be associated cracking
- Keep cap on bottle when not in use.
- Lot No. and EXP date: see label, bottom container or box.
Inactive ingredients
Citric Acid, Glycerin, Propylene Glycol, Sodium Citrate, Sodium Lauryl Sulfate, Tartaric Acid
Principal Display Panel Bottle Label 0.5 FL OZ
Goodsense NDC 50804-341-51
Ear Wax Removal drops
Carbamide Peroxide 6.5%
0.5 FL OZ (15ml)