ACID REDUCER - ranitidine tablet
Dolgencorp, LLC
----------
Uses
- relieves heartburn associated with acid indigestion and sour stomach
- prevents heartburn associated with acid indigestion and sour stomach brought on by eating or drinking certain foods and beverages
Do not use
- if you have trouble or pain swallowing food, vomiting with blood, or bloody or black stools. These may be signs of a serious condition. See your doctor.
- with other acid reducers
- if you have kidney disease, except under the advice and supervision of a doctor
Ask a doctor before use if you have
- had heartburn over 3 months. This may be a sign of a more serious condition.
- heartburn with lightheadedness, sweating or dizziness
- chest pain or shoulder pain with shortness of breath; sweating; pain spreading to arms, neck or shoulders; or lightheadedness
- frequent chest pain
- frequent wheezing, particularly with heartburn
- unexplained weight loss
- nausea or vomiting
- stomach pain
Stop use and ask a doctor if
- your heartburn continues or worsens
- you need to take this product for more than 14 days
Keep out of reach of children.
In case of overdose, get medical help or contact a Poison Control Center (1-800-222-1222) right away.
Directions
- adults and children 12 years and over:
- to relieve symptoms, swallow 1 tablet with a glass of water
- to prevent symptoms, swallow 1 tablet with a glass of water 30 to 60 minutes before eating food or drinking beverages that cause heartburn
- can be used up to twice daily (do not take more than 2 tablets in 24 hours)
- children under 12 years: ask a doctor
Other information
-
TAMPER EVIDENT: Do Not Use If The Carton Or Printed Foil Under Cap Is Open or Torn
- store at 20° to 25°C (68° to 77°F)
- avoid excessive heat or humidity
- this product is sugar free
- USP Dissolution Test Pending
Inactive ingredients
croscarmellose sodium, hypromellose, magnesium stearate, microcrystalline cellulose, titanium dioxide, and triacetin
Questions?
call 1-888-309-9030
Read the directions, consumer information leaflet and warnings before use. Keep the carton. It contains important information.
Consumer Information
What you should know about
MAXIMUM STRENGTH
Ranitidine Tablets USP, 150 mg
ACID REDUCER
(Please read all of this information before taking MAXIMUM STRENGTH
Ranitidine Tablets 150 mg. Save this leaflet for future reference.)
What are MAXIMUM STRENGTH Ranitidine Tablets 150 mg?
- MAXIMUM STRENGTH Ranitidine Tablets 150 mg contain 150 mg of ranitidine (as ranitidine hydrochloride USP, 167.414 mg), a medicine that doctors have prescribed more than 200 million times worldwide.
Excellent Safety Record
- The ingredient in MAXIMUM STRENGTH Ranitidine Tablets 150 mg, ranitidine, has been prescribed by doctors for years to treat millions of patients safely and effectively. The active ingredient in MAXIMUM STRENGTH Ranitidine Tablets 150 mg has been taken safely with many frequently prescribed medications.
- MAXIMUM STRENGTH Ranitidine Tablets 150 mg are sugar free.
What symptoms do MAXIMUM STRENGTH Ranitidine Tablets 150 mg relieve and prevent?
- MAXIMUM STRENGTH Ranitidine Tablets 150 mg relieve and prevent heartburn associated with acid indigestion and sour stomach.
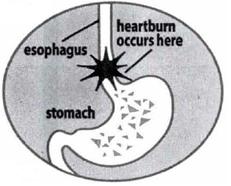
- Certain foods or beverages, and even lying down to sleep can cause heartburn associated with acid indigestion and sour stomach. It is normal for the stomach to produce acid, especially after consuming food or beverages. However, acid in the wrong place, such as the esophagus, or too much acid, can cause burning pain and discomfort.
How should I take MAXIMUM STRENGTH Ranitidine Tablets 150 mg?
- To relieve symptoms, swallow 1 tablet with a glass of water.
- To prevent symptoms, swallow 1 tablet with a glass of water 30 to 60 minutes before eating food or drinking beverages that cause heartburn.
- This medicine can be used up to twice daily (do not take more than 2 tablets in 24 hours).
- MAXIMUM STRENGTH Ranitidine Tablets 150 mg should not be given to children under 12 years old unless directed by a doctor.
- Allergy alert: Do not use if you are allergic to ranitidine or other acid reducers
How do MAXIMUM STRENGTH Ranitidine Tablets 150 mg work?
- MAXIMUM STRENGTH Ranitidine Tablets 150 mg reduce the production of stomach acid. This is what makes MAXIMUM STRENGTH Ranitidine Tablets 150 mg different from antacids, which neutralize the acid already in your stomach. Antacids do not reduce the production of acid.
Tips for managing heartburn
- Do not lie flat or bend over soon after eating
- Do not eat late at night, or just before bedtime
- Certain foods or drinks are more likely to cause heartburn, such as rich, spicy, fatty, and fried foods, chocolate, caffeine, alcohol, even some fruits and vegetables
- Eat slowly and do not eat big meals
- If you are overweight, lose weight
- If you smoke, quit smoking
- Raise the head of your bed
- Wear loose fitting clothing around your stomach
When should I see a doctor?
-
Do not use
- if you have trouble or pain swallowing food, vomiting with blood, or bloody or black stools. These may be signs of a serious condition. See your doctor.
- with other acid reducers
- if you have kidney disease, except under the advice and supervision of a doctor
-
Ask a doctor before use if you have
- had heartburn over 3 months. This may be a sign of a more serious condition.
- heartburn with lightheadedness, sweating or dizziness
- chest pain or shoulder pain with shortness of breath; sweating; pain spreading to arms, neck or shoulders; or lightheadedness
- frequent chest pain
- frequent wheezing, particularly with heartburn
- unexplained weight loss
- nausea or vomiting
- stomach pain
-
Stop use and ask a doctor if
- your heartburn continues or worsens
- you need to take this product for more than 14 days
- If pregnant or breast-feeding, ask a healthcare professional before use.
- Keep out of reach of children. In case of overdose, get medical help or contact a Poison Control Center (1-800-222-1222) right away.
Questions? Call 1-888-309-9030
BOTTLES: Do not use if the carton or printed foil under cap is open or torn.
DISTRIBUTED BY:
DOLGENCORP, LLC
100 MISSION RIDGE,
GOODLETTSVILLE, TN 37072
MADE IN INDIA
Revised: 05/ 2019
PACKAGE LABEL-PRINCIPAL DISPLAY PANEL - 150 mg Container (24 Tablets Bottle)
DG™ health
Maximum Strength Acid Reducer
Ranitidine Tablets 150 mg
Acid Reducer
PREVENTS & RELIEVES HEARTBURN
Associated with
Acid Indigestion
and Sour Stomach
8 Tablets (8 Doses)

PACKAGE LABEL-PRINCIPAL DISPLAY PANEL - 150 mg Container Carton (24's Tablets)
DG™ health
Compare to the
active Ingredient
of Zantac 1509®*
Maximum Strength
Acid
Reducer
Ranitidine Tablets 150 mg
Acid Reducer
PREVENTS & RELIEVES
HEARTBURN
Associated with
Acid Indigestion
and Sour Stomach
150 mg 8 Tablets (8 Doses)

| ACID REDUCER
ranitidine tablet |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - Dolgencorp, LLC (068331990) |
| Registrant - Aurohealth LLC (078728447) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Aurobindo Pharma Limited | 650381903 | ANALYSIS(55910-092) , MANUFACTURE(55910-092) | |