INFANTS RELIEF NON-STAINING FORMULA- dimethicone 410 liquid
CVS
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
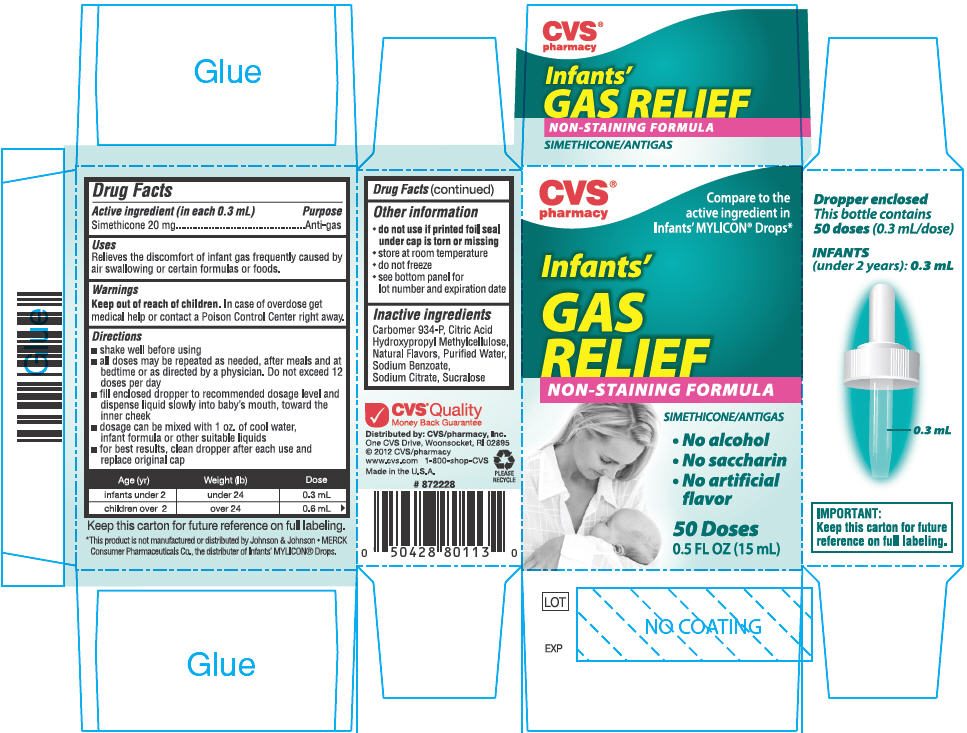
Active ingredient (in each 0.3 mL)
Simethicone 20 mg
Uses
Relieves the discomfort of infant gas frequently caused by air swallowing or certain formulas or foods.
Warnings
Keep out of reach of children. In case of overdose get medical help or contact a Poison Control Center right away.
Directions
- shake well before using
- all doses may be repeated as needed, after meals and at bedtime or as directed by a physician. Do not exceed 12 doses per day
- fill enclosed dropper to recommended dosage level and dispense liquid slowly into baby's mouth, toward the inner cheek
- dosage can be mixed with 1 oz. of cool water, infant formula or other suitable liquids
- for best results, clean dropper after each use and replace original cap
| Age (yr) | Weight (lb) | Dose |
| infants under 2 | under 24 | 0.3 mL |
| children over 2 | over 24 | 0.6 mL |
Other information
-
do not use if printed foil seal under cap is torn or missing
- store at room temperature
- do not freeze
- see bottom panel for lot number and expiration date
Inactive ingredients
Carbomer 934-P, Citric Acid, Hydroxypropyl Methylcellulose, Natural Flavors, Purified Water, Sodium Benzoate, Sodium Citrate, Sucralose
Distributed by: CVS/pharmacy, Inc.
One CVS Drive, Woonsocket, RI 02895
PRINCIPAL DISPLAY PANEL - 15 mL Bottle Carton
CVS®
pharmacy
Compare to the
active ingredient in
Infants' MYLICON® Drops*
Infants'
GAS
RELIEF
NON-STAINING FORMULA
SIMETHICONE/ANTIGAS
• No alcohol
• No saccharin
• No artificial
flavor
50 Doses
0.5 FL OZ (15 mL)