URISED- methenamine, phenyl salicylate, methylene blue, benzoic acid, atropine sulfate , hyoscyamine tablet
Key Therapeutics
Disclaimer: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
----------
Urised
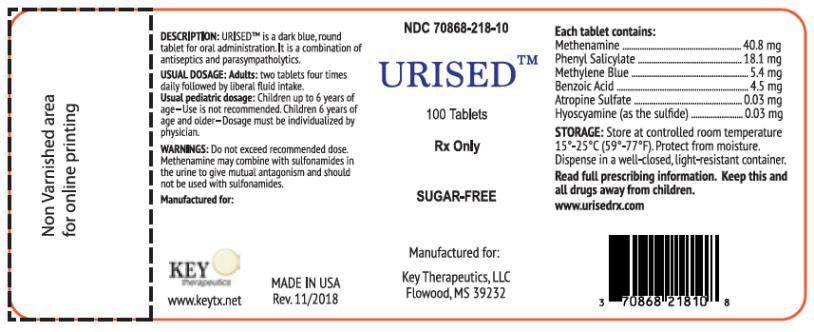
DESCRIPTION
U R I S E D™ is a dark blue, round, tablet for oral administration. It is a combination of antiseptics (Methenamine, Methylene Blue, Phenyl Salicylate, Benzoic Acid) and parasympatholytics (Atropine Sulfate, Hyoscyamine).
Each tablet contains: Methenamine 40.8 mg, Phenyl Salicylate 18.1 mg, Methylene Blue 5.4 mg, Benzoic Acid 4.5 mg, Atropine Sulfate 0.03 mg and Hyoscyamine (as the sulfate) 0.03 mg.
CLINICAL PHARMACOLOGY
Methenamine itself does not have antiseptic, irritant, or toxic properties in the urine. Methenamine, in an acid urine (pH 6 or below), hydrolyzes into formaldehyde within the urinary tract providing mild antiseptic activity. When given as directed and the daily urine volume is 1000 to 1500 mL, a daily dose of 2 grams will yield a urinary concentration of 18-60 mcg/mL of free formaldehyde in the urine. This is more than the minimal inhibitory dose of formaldehyde which must be available for most urinary tract pathogens. Methenamine is readily absorbed from the gastrointestinal tract and is rapidly excreted almost entirely in the urine. Methylene Blue and Benzoic Acid are mild but effective antiseptics which contribute to the antiseptic properties of Methenamine. Phenyl Salicylate is a mild analgesic and antipyretic with weak antiseptic activity. All of these compounds are readily absorbed from the gastrointestinal tract and excreted in the urine. Through parasympatholytic actions, atropine and hyoscyamine relax smooth muscle spasms resulting from parasympathic stimulation.
INDICATIONS AND USAGE
U R I S E D™ is indicated for the relief of discomfort of the lower urinary tract caused by hypermotility resulting from inflammation or diagnostic procedures and in the treatment of cystitis, urethritis, and trigonitis when caused by organisms which maintain or produce an acid urine and are susceptible to formaldehyde.
CONTRAINDICATIONS
Glaucoma, urinary bladder neck obstruction, pyloric or duodenal obstruction, or cardiospasm. Hypersensitivity to any of the ingredients.
WARNINGS
Do not exceed recommended dose. Methenamine may combine with sulfonamides in the urine to give mutual antagonism and should not be used with sulfonamides.
PRECAUTIONS
General:
Administer with caution to persons with known idiosyncrasy to atropine-like compounds and to patients suffering from cardiac disease. Bacteriological studies of the urine may be helpful in following the patient response. Methylene Blue interferes with the analysis for some urinary components such as free formaldehyde. In acid urine Methenamine breaks down into formaldehyde, which may form an insoluble precipitate with certain sulfonamides and may also increase the danger of crystalluria; concurrent use is not recommended. No known long-term animal studies have been performed to evaluate carcinogenic potential. The precautions related to drug interaction, diagnostic interference, medical problems and side effects to use of belladonna alkaloids, should be observed.
Information for Patients:
You should avoid using drugs and/or foods that produce alkaline urine while taking this medicine. If you are taking any anti-infective medications containing a sulfonamide, check with your doctor before using U R I S E D™. While taking this medicine, your urine may become blue to blue-green and the feces may be discolored as a result of excretion of Methylene Blue, so care should be taken to avoid staining clothing or other items. To avoid Methylene Blue stains on skin, mouth or teeth, make sure your hands are dry and that the tablets are swallowed quickly with liberal fluid intake.
Pregnancy Category C:
Animal reproduction studies have not been conducted with U R I S E D™ tablets. It is also not known whether U R I S E D™ tablets can cause fetal harm when administered to a pregnant woman or can affect reproduction capacity. U R I S E D™ tablets should be given to a pregnant woman only if clearly needed.
ADVERSE REACTIONS
Prolonged use may result in a generalized skin rash, pronounced dryness of the mouth, flushing, difficulty in initiating micturition, rapid pulse, dizziness or blurring of vision. If any of these reactions occurs, discontinue use immediately. Acute urinary retention may be precipitated in prostatic hypertrophy. See “OVERDOSE”.
DRUG ABUSE AND DEPENDENCE
A dependence on the use of U R I S E D™ has not been reported and due to the nature of its ingredients, abuse of U R I S E D™ is not expected.
OVERDOSE
By exceeding the recommended dosage of U R I S E D™, symptomology related to the overdose of its individual active ingredients may be expected as follows:
Atropine Sulfate, Hyoscyamine: Symptoms associated with an overdosage of U R I S E D™ will most probably be manifested in the symptoms related to overdosage of the alkaloids Atropine Sulfate and Hyoscyamine. Such symptoms as dryness of mucous membranes; dilation of pupils; hot, dry, flushed skin; hyperpyrexia; tachycardia; palpitations; elevated blood pressure; coma; circulatory collapse and death from respiratory failure can occur due to overdosage of these alkaloids.
Methenamine: If large amounts of the drug (2-8 gm daily) are used over extended periods (3-4 weeks), bladder and gastrointestinal irritation, painful and frequent micturition, albuminuria and gross hematuria may be expected.
Methylene Blue: Symptoms of Methylene Blue overdosage associated with the overdosage of U R I S E D™ are not expected to be discernible from those associated with the other active ingredients in U R I S E D™.
Benzoic Acid: Symptoms of Benzoic Acid overdosage associated with the overdosage of U R I S E D™ are not expected to be discernable from those associated with the other active ingredients in U R I S E D™.
Phenyl Salicylate: Symptoms of Phenyl Salicylate overdosage include burning pain in throat and mouth, white necrotic lesions in the mouth, abdominal pain, vomiting, bloody diarrhea, pallor, sweating, weakness, headache, dizziness and tinnitus. The symptoms, however, are not expected to be discernable from those associated with the other active ingredients in U R I S E D™.
*These statements have not been evaluated by the Food and Drug Administration. This product is not included in the Orange Book.
DOSAGE AND ADMINISTRATION
Adults: Two tablets four times daily. See “PRECAUTIONS.”
Usual pediatric dosage: Children up to 6 years of age—Use is not recommended. Children 6 years of age and older—Dosage must be individualized by physician.
STORAGE:
Store at controlled room temperature 15°-25°C (59°-77°F). Protect from moisture. Dispense in well-closed, light-resistant container.
KEEP THIS PRODUCT OUT OF THE REACH OF CHILDREN.
MANUFACTURED FOR:
KEY THERAPEUTICS, LLC.
FLOWOOD, MS 39232
www.keytx.net
MADE IN USA
© Rev. 11/18
U R I S E D™ is a trademark licensed to Key Therapeutics.
| URISED
methenamine, phenyl salicylate, methylene blue, benzoic acid, atropine sulfate , hyoscyamine tablet |
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
| Labeler - Key Therapeutics (080318791) |