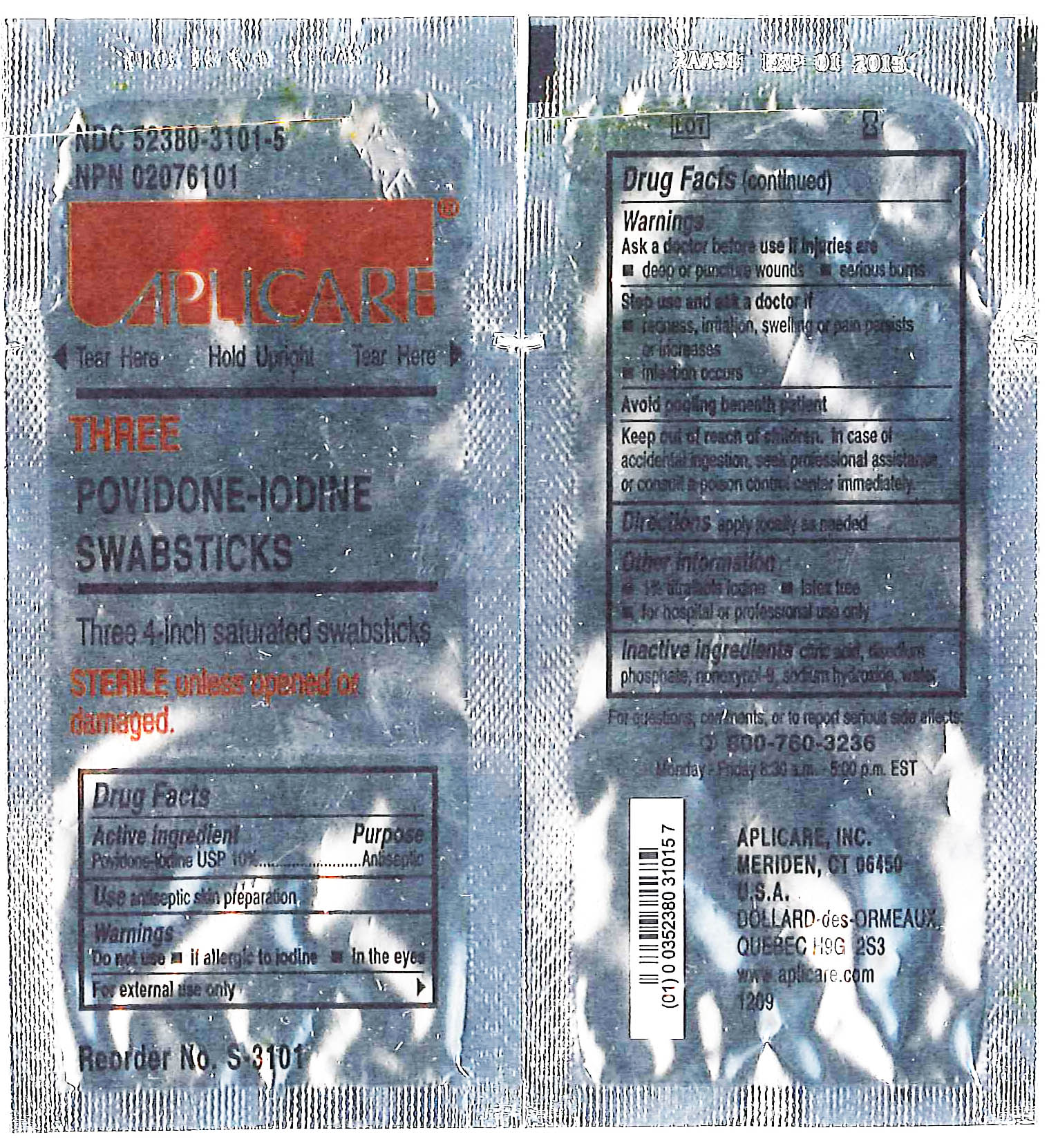
Drug Facts
APLICARE THREE POVIDONE-IODINE SWABSTICKS
[Aplicare, Inc.]
Three 4-inch saturated swabsticks
Povidone-iodine 10%
Antiseptic
Warnings
Do not use
- if allergic to iodine
- in the eyes
For external use only
Ask a doctor before use if injuries are
- deep or puncture wounds
- serious burns
Stop use and ask a doctor if
- redness, irritation, swelling or pain persists or increases
- infection occurs
Avoid pooling beneath patient
Keep out of reach of children. In case of accidental ingestion, seek professional assistance or consult a poison control center immediately.