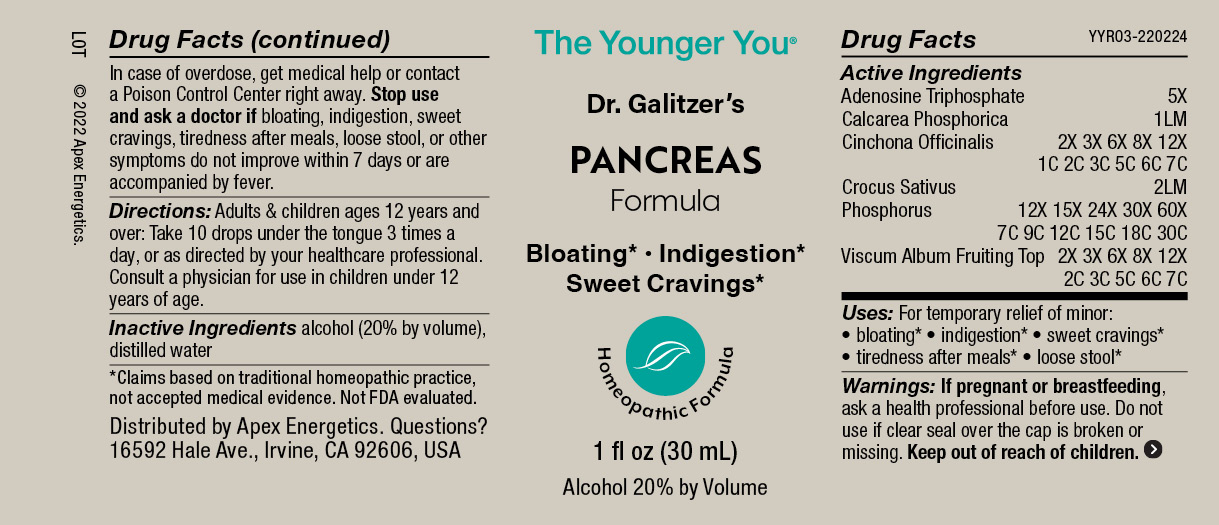
PANCREAS FORMULA- adenosine triphosphate, tribasic calcium phosphate, cinchona officinalis bark, saffron, phosphorus, viscum album fruiting top solution/ drops
Apex Energetics Inc.
Disclaimer: This homeopathic product has not been evaluated by the Food and Drug Administration for safety or efficacy. FDA is not aware of scientific evidence to support homeopathy as effective.
----------
|
Active Ingredients
|
|
Adenosine Triphosphate
|
5X
|
|
Calcarea Phosphorica
|
1LM
|
|
Cinchona Officinalis
|
2X 3X 6X 8X 12X 1C 2C 3C 5C 6C 7C
|
|
Crocus Sativus
|
2LM
|
|
Phosphorus
|
12X 15X 24X 30X 60X 7C 9C 12C 15C 18C 30C
|
|
Viscum Album Fruiting Top
|
2X 3X 6X 8X 12X 2C 3C 5C 6C 7C
|
Uses:
For temporary relief of minor:
bloating*
indigestion*
sweet cravings*
tiredness after meals*
loose stool*
*Claims based on traditional homeopathic practice, not accepted medical evidence. Not FDA evaluated.
Warnings:
If pregnant or breastfeeding, ask a health professional before use.
Do not use if clear seal over the cap is broken or missing.
Keep out of reach of children. In case of overdose, get medical help or contact a Poison Control Center right away.
Stop use and ask a doctor if bloating, indigestion, sweet cravings, tiredness after meals, loose stool, or other symptoms do not improve within 7 days or are accompanied by fever.
Directions:
Adults & children ages 12 years and over: Take 10 drops under the tongue 3 times a day, or as directed by your healthcare professional. Consult a physician for use in children under 12 years of age.
alcohol (20% by volume), distilled water
Distributed by Apex Energetics. Questions?
16592 Hale Ave., Irvine, CA 92606, USA
The Younger You®
Dr. Galitzer’s
PANCREAS
Formula
Bloating* Indigestion*
Sweet Cravings*
Homeopathic Formula
1 fl oz (30 mL)
Alcohol 20% by Volume
Apex Energetics Inc.
